Disturbances in phosphate levels are common in critically ill children,1 and have been associated with malnutrition, refeeding syndrome,2–4 parenteral nutrition (PN),5 and the use of diuretics and vasoactive drugs.1,4,6 Some studies have reported an association between hypophosphatemia and hyperphosphatemia and mortality, severity status1,2 and the duration of admission to the Pediatric Intensive Care Unit (PICU).1,3
Following approval by the Research Ethics Committee, a retrospective, single-center, observational study was carried out. The study aimed to analyze the related factors and the impact of phosphate disturbances in children admitted to the PICU.
We included patients for whom phosphate levels were measured during admission to the PICU over a period of 12 months. Patient age, sex, date of admission and discharge, anthropometric data, personal history, clinical severity scales and blood phosphate, calcium, creatinine, albumin, parathormone (PTH), magnesium and lactate values were recorded at different timepoints: T0: day 1 and day 2; T1: days 3–7; T2: days 8–14; and Tdischarge. Treatment with phosphate, drugs, blood products, renal replacement therapy (RRT), enteral nutrition (EN) or PN, invasive mechanical ventilation (IMV) or noninvasive mechanical ventilation (NIMV), extracorporeal membrane oxygenation (ECMO) and mortality were also recorded. The normal range of phosphatemia was between 4−7 mg/dl. Continuous variables were analyzed using the Mann–Whitney U test and the median test, categorical variables using the Chi-square test and Fisher’s exact test, and correlation analysis using Pearson's correlation coefficient (〉). Statistical significance was considered for p < 0.05. First, a univariate analysis was performed, followed by a multivariate logistic regression analysis of the factors related to phosphate disturbances.
Of the 370 PICU admissions, 232 patients were included in the analysis (41.8% female; see Table 1 E of the Supplementary Material). The median length of stay in the PICU was 3 days (IQR: 2–9). Ten patients died (4.3%). As in healthy children, age and serum phosphate levels showed an inverse proportional relationship. Surgical patients had higher phosphate levels upon admission than the rest of the patients (5.6 [4.7–6.8] vs. 4.4 mg/dl [3.7–5.1]; p < 0.001).
Factors associated with phosphatemia disturbances on admission and during admission to the PICU.
| Hyperphosphatemia on admission | Hyperphosphatemia during admission | Hypophosphatemia on admission | Hypophosphatemia during admission | |||||
|---|---|---|---|---|---|---|---|---|
| n/N (%) | p-Value | n/N (%) | p-Value | n/N (%) | p-Value | n/N (%) | p-Value | |
| Sex | ||||||||
| Female | 14/88 (15.9%) | 0.4 | 22/97 (22.7%) | 0.856 | 19/88 (21.6%) | 0.866 | 41/97 (42.3%) | 0.375 |
| Male | 15/126 (11.9%) | 32/135 (23.7%) | 26/126 (20.6%) | 65/135 (48.1%) | ||||
| Post-surgical admission | ||||||||
| Yes | 27/114 (23.7%) | <0.001 | 33/117 (28.2%) | 0.073 | 13/114 (11.4%) | <0.001 | 53/117 (45.3%) | 0.904 |
| No | 2/100 (2%) | 21/115 (18.3%) | 32/100 (32%) | 53/115 (46.1%) | ||||
| IMV | ||||||||
| Yes | 18/75 (24%) | 0.001 | 41/86 (47.7%) | <0.01 | 13/75 (17.3%) | 0.33 | 39/86 (45.3%) | 0.936 |
| No | 11/139 (7.9%) | 13/146 (8.9%) | 32/139 (23%) | 67/146 (45.9%) | ||||
| NIMV | ||||||||
| Yes | 21/82 (25.6%) | <0.001 | 41/94 (43.6%) | <0.001 | 10/82 (12.2%) | 0.012 | 41/94 (43.6%) | 0.601 |
| No | 8/132 (6.1%) | 13/138 (9.4%) | 35/132 (26.5%) | 65/138 (47.1%) | ||||
| Artificial nutrition | ||||||||
| Yes | 24/87 (27.6%) | <0.001 | 47/102 (46.1%) | <0.001 | 14/87 (16.1%) | 0.142 | 43/102 (42.2%) | 0.339 |
| No | 5/127 (3.9%) | 7/130 (5.4%) | 31/127 (24.4%) | 63/130 (48.5%) | ||||
| Parenteral nutrition | ||||||||
| Yes | 1/21 (4.8%) | 0.213 | 7/24 (29.2%) | 0.444 | 8/21 (38.1%) | 0.045 | 12/24 (50%) | 0.637 |
| No | 28/192 (14.6%) | 46/207 (22.2%) | 37/192 (19.3%) | 93/207 (44.9%) | ||||
| RRT | ||||||||
| Yes | 6/17 (35.3%) | 0.006 | 16/19 (84.2%) | <0.001 | 3/17 (17.6%) | 0.721 | 11/19 (57.9%) | 0.265 |
| No | 23/197 (11.7%) | 38/213 (17.8%) | 42/197 (21.3%) | 95/213 (44.6%) | ||||
| ECMO | ||||||||
| Yes | 1/9 (11.1%) | 0.827 | 8/11 (72.7%) | <0.001 | 1/9 (11.1%) | 0.456 | 5/11 (45.5%) | 0.987 |
| No | 28/205 (13.7%) | 46/221 (20.8%) | 44/205 (21.5%) | 101/221 (45.7%) | ||||
| Cardiac arrest | ||||||||
| Yes | 2/7 (28.6%) | 0.236 | 4/8 (50%) | 0.05 | 2/7 (28.6%) | 0.605 | 3/8 (37.5%) | 0.66 |
| No | 26/200 (13%) | 45/216 (20.8%) | 41/200 (20.5%) | 98/216 (45.4%) | ||||
| Mortality | ||||||||
| Yes | 1/8 (12.5%) | 0.929 | 5/10 (50%) | 0.041 | 5/8 (72.5%) | 0.003 | 3/10 (30%) | 0.309 |
| No | 28/206 (13.6%) | 49/222 (22.1%) | 40/206 (19.4%) | 103/222 (46.4%) | ||||
ECMO: extracorporeal membrane oxygenation; CA: cardiac arrest; RRT: renal replacement therapy; PICU: Pediatric Intensive Care Unit; IMV: invasive mechanical ventilation; NIMV: noninvasive mechanical ventilation.
Fig. 1E of the Supplementary material shows the evolution of the phosphate, calcium and creatinine values, and the percentages of hypophosphatemia and hyperphosphatemia. In surgical patients, the phosphate levels were higher on admission and subsequently decreased. This pattern was not observed in medical patients (see Fig. 2E of the Supplementary material).
Tables 1 and 2 show the variables associated with hyperphosphatemia and hypophosphatemia in the univariate and multivariate analyses, respectively.
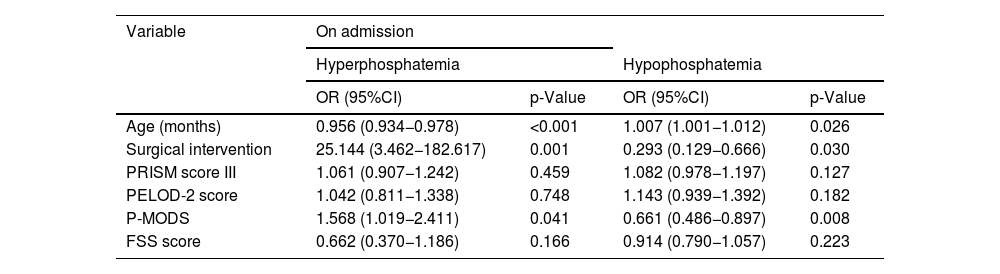
Multivariate analysis of factors related to phosphate disturbances on admission and during admission to the PICU.
| Variable | On admission | |||
|---|---|---|---|---|
| Hyperphosphatemia | Hypophosphatemia | |||
| OR (95%CI) | p-Value | OR (95%CI) | p-Value | |
| Age (months) | 0.956 (0.934−0.978) | <0.001 | 1.007 (1.001−1.012) | 0.026 |
| Surgical intervention | 25.144 (3.462−182.617) | 0.001 | 0.293 (0.129−0.666) | 0.030 |
| PRISM score III | 1.061 (0.907−1.242) | 0.459 | 1.082 (0.978−1.197) | 0.127 |
| PELOD-2 score | 1.042 (0.811−1.338) | 0.748 | 1.143 (0.939−1.392) | 0.182 |
| P-MODS | 1.568 (1.019−2.411) | 0.041 | 0.661 (0.486−0.897) | 0.008 |
| FSS score | 0.662 (0.370−1.186) | 0.166 | 0.914 (0.790−1.057) | 0.223 |
| Variable | During admission | |||
|---|---|---|---|---|
| Hyperphosphatemia | Hypophosphatemia | |||
| OR (95%CI) | p-Value | OR (95%CI) | p-Value | |
| Age (months) | 0.984 (0.974−0.993) | <0.001 | 1.008 (1.003−1.012) | 0.001 |
| Surgery | 7.585 (2.466−23.334) | <0.001 | 0.981 (0.556−1.733) | 0.948 |
| Length of stay | 1.026 (1.000−1.052) | 0.048 | 1.001 (0.988−1.015) | 0.840 |
| NIMV | 4.552 (1.572−13.178) | 0.005 | 0.873 (0.450−1.694) | 0.688 |
| Artificial nutrition | 4.381 (1.393−13.784) | 0.012 | 1.100 (0.531−2.276) | 0.798 |
| Parenteral nutrition | 0.109 (0.010−1.227) | 0.073 | 0.977 (0.364−2.623) | 0.963 |
| RRT | 38.973 (2.694−563.897) | 0.007 | 1.101 (0.292−4.153) | 0.887 |
95%CI: 95% confidence interval; Artificial nutrition: nasogastric tube/transpyloric tube/gastrostomy; OR: odds ratio; PELOD: Pediatric Logistic Organ Dysfunction; FSS: Functional Status Scale; P-MODS: Pediatric Multiple Organ Dysfunction Score; PRISM-III: Pediatric Risk of Mortality; IQR: interquartile range; RRT: renal replacement therapy; PICU: Pediatric Intensive Care Unit; NIMV: noninvasive mechanical ventilation.
As shown in Table 1, children with hyperphosphatemia at and during admission had lower ages and weights, higher severity scores and longer lengths of stay in the PICU. Fifty percent of the deceased patients developed hyperphosphatemia during their admission compared to 20.8% of the survivors (p = 0.041).
Hyperphosphatemia occurred more frequently in patients treated with corticosteroids (33.3% vs. 19.8%; p = 0.030), vasoactive drugs (36.5% vs. 10.5%; p < 0.001), furosemide (39.2% vs. 6.4%; p < 0.001), acetazolamide (50% vs. 22%; p = 0.017), vitamin D (47.6% vs. 21.2%; p = 0.006) and iron (35% vs. 20.2%; p = 0.043).
Multivariate analysis revealed that hyperphosphatemia at admission was associated with younger age, surgical admission and a higher Pediatric Multiple Organ Dysfunction Score (P-MODS). Hyperphosphatemia during admission was associated with younger age, surgical admission, noninvasive mechanical ventilation (NIMV), artificial nutrition, renal replacement therapy (RRT), and a longer duration of admission, as has been described in adults7 (Table 2).
In patients undergoing RRT, phosphorus levels decrease if dialysis fluids are not supplemented with phosphate,8 but our patients received phosphate supplements in their RRT fluids. Perhaps because of this, they more frequently presented hyperphosphatemia.6
Hyperphosphatemia was more frequent in patients who required mechanical ventilation, which coincides with the observations of other studies. No pathophysiological reason has been found to explain this association. Patients with hyperphosphatemia had higher severity scores, suggesting that hyperphosphatemia could be a marker of severity in critically ill children.6,7,9
HypophosphatemiaHypophosphatemia occurred more frequently than hyperphosphatemia, which aligns with data found in the literature.6 The main causes of hypophosphatemia are intracellular redistribution due to respiratory alkalosis, decreased intake, or hemodilution when phosphate-free fluids are used.3
Hypophosphatemia was more prevalent among medical patients, those subjected to NIMV and PN, and those who died upon admission to the PICU. Patients presenting hypophosphatemia at and during admission were older and had a greater body weight (Table 1).
Some studies have found an association between hypophosphatemia and the duration of mechanical ventilation.1,6 The univariate study showed an association between hypophosphatemia upon admission and NIMV; however, this association was not confirmed in the multivariate study. We found no association between hypophosphatemia and PN, corticosteroids, furosemide or vasoactive drugs, as previously reported by other authors.1,10
The univariate analysis revealed an association between hypophosphatemia at admission and mortality. A total of 72.5% of the deceased patients had hypophosphatemia at admission compared to 19.4% of the survivors; p = 0.003. However, this association was not maintained in the multivariate analysis.
In the multivariate analysis, hypophosphatemia at admission was associated with older age, medical causes of admission and lower P-MODS scores. Hypophosphatemia during admission was only associated with older age (Table 2).
The main limitations of our study are its retrospective design, which precludes the establishment of causal relationships, and the fact that phosphate levels were not determined for some patients, which may cause the incidence of the alterations to be overestimated.
In conclusion, phosphate disturbances are frequent in children admitted to the PICU. Hyperphosphatemia was more prevalent in surgical patients, smaller patients, children subjected to NIMV, patients receiving artificial nutrition and patients with RRT. It was also associated with a longer duration of admission. Hypophosphatemia upon admission and hyperphosphatemia during admission were more prevalent in deceased patients, though a causal relationship could not be established.
CRediT authorship contribution statementAll authors have made substantial contributions in each of the following areas:
Nuria del Amo-Carramiñana, Paula Lasarte-Merino, Celia Pascual-Alonso and Rafael González-Cortés: acquisition, analysis and interpretation of the data, drafting of the article, and final approval of the manuscript.
Jesús López-Herce: conception and design of the study, acquisition, analysis and interpretation of the data, drafting of the article, critical review of the intellectual content, and the final approval of the manuscript.
Declaration of generative AI and AI-assisted technologies in the writing processThe authors declare that they have not used artificial intelligence for any activities related to the study or in the writing of the manuscript.
FinancingThe authors have not received any funding to conduct this study.
The authors declare that they have no conflicts of interest.
We would like to acknowledge the physicians and nurses of the Department of Pediatric Intensive Care Medicine at Hospital General Universitario Gregorio Marañón in Madrid, Spain, for their help in data collection and patient management.