Spontaneous coronary artery dissection (SCAD) is a rare cause for the acute coronary syndrome (ACS). Since its first description 88 years ago, the information available on this entity has always been very scarce and based on ambiguous data from a plethora of anecdotal clinical cases and small retrospective series of patients. However, over the last decade, important advances have been made in our understanding of this interesting condition.1–3 Today, we have new information from some relatively large prospective series and well-designed registries at national level in different countries.1–5 However, scientific evidence on this condition is still scarce compared to the information available on other more common cardiovascular diseases. This means that, to this point, the publication of conventional clinical practice guidelines is still far away. However, two important consensus documents were published last year by experts in the management of SCAD1,2 that were coordinated from both sides of the Atlantic. There is no doubt that we are at the dawn of a new era regarding our knowledge of this entity that should help us improve its diagnosis and treatment. But what have we learned over the last few years about SCAD?
SCAD is defined as a separation of the layers of the coronary artery wall with formation of 2 different lumens.1,2 These lumens can be communicated between the two (true lumen and false lumen) or not (intramural hematoma). Coronary artery dissections associated with vascular atherosclerotic or iatrogenic plaque complications are not included in the current definition of SCAD.1–5
In the first place, we still do not know the etiology or physiopathology of this entity. As a matter of fact, the two classic hypotheses still stand today: 1) intimal rupture due to an increased pressure leading to the formation of false lumen due to spreads, and 2) intraparietal bleeding (due to rupture of vasa vasorum) with formation of intramural hematoma that can progress into intimal tears and communication with the true lumen.1–5 Some recent evidence may support this second hypothesis (from the outside to the inside). In any case, the compromise of the true lumen determines the ischemic signs.
Secondly, we know that the most classic clinical profile is exceptionally rare: very young woman without coronary risk factors who had a labor related acute myocardial infarction. We have learned that SCAD often occurs in middle-aged women (4th–5th decades of life) with some coronary risk factor who have an ACS.1–5 In half of the patients, physical or emotional stress can be the triggering cause. Unfortunately, the clinical signs are undistinguishable from those of the ACS due to coronary atherosclerosis, although the therapeutic approach is very different. The peripartum type is exceptionally rare (<3%), a situation that seems to be associated with a worse prognosis. On the other hand, the multiple conditions traditionally associated with SCAD (systemic inflammatory disorders, colagenosis, etc.) seem to affect a very low number of patients (<5%) according to the most important series reported to this date. However, its association with fibromuscular dysplasia (FMD) has been well-established and confirmed in several independent series with variable prevalence (25%–85%) based on the thoroughness and diagnostic method used for screening.1,2,6 The etiology of FMD is also unknown, it is not atherosclerotic or inflammatory, and it affects mostly major arteries (iliac, renal, carotid arteries). Although this association does not imply causality, it is tempting to speculate on a common underlying substrate of vulnerability or vascular frailty.6 Also, it has been suggested that FMD may affect the coronary tree, although the evidence available on this regard has been highly questioned. Finally, the association of familial cases is anecdotal. However, damage to the PHACTR1/EDN1 genetic locus (genotype rs9349379) has been recently described in patients with SCAD, which, oddly enough, is also involved in FMD.7 Although these new data are still preliminary, the study of a possible underlying genetic base of the disease is very interesting.
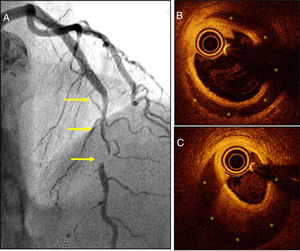
Thirdly, we have learned that the prevalence of SCAD in patients with ACS is much higher compared to the one traditionally described.8 The greater clinical suspicion has been essential to improve our diagnostic capabilities. Therefore, the systemic use of high-sensitivity cardiac troponin and performing very early coronary angiographies in women with ACS have been able to diagnose many cases that used to go misdiagnosed.1–5,8 Now we know that the traditional image of angiographic dissection (a typical image of double lumen or intimal flap) is actually found in one third of the patients only. In most cases, diffuse lesions are found on the angiography, some of them very typical ones, or else focal stenoses (that imitate atherosclerotic lesions) (Fig. 1A). The presence of significant coronary tortuosity is also common.1–5,8 High clinical suspicion allows us to diagnose these cases. Thus, it has been suggested that SCAD could be the cause of one third of all ACSs suffered by women ≤50 years of age.4 On the other hand, intracoronary imaging modalities (coronary ultrasound and, more recently, optical coherence tomography [with an amazing spatial resolution: 15μm]) have allowed us to confirm the diagnosis in angiographically suspicious cases.9 Also, these tomographic imaging modalities allow us to study the true spread and severity of coronary disease in great detail by providing spectacular images of the true lumen, the false lumen, and the entry (or of the intramural hematoma that compresses the coronary lumen)9 (Fig. 1B,C). Most cases reveal that separation does not occur at intima layer level but between the tunica media external third portion and the tunica adventitia, which generates a relatively thick «intima-media» membrane as necropsy findings have always revealed. Also, these new imaging modalities have been very useful to show us that, in most patients, there is a spontaneous complete vascular repair (restitutio ad integrum) at the follow-up that recovers the parietal architecture (tunica intima, tunica media, and tunica adventitia) that ends up looking completely normal.9
A) Patient with acute coronary syndrome (ACS) with a severe and long angiographical lesion (without double lumen) typical of a spontaneous coronary artery dissection (SCAD) in the left anterior descending coronary artery middle segment (arrows). B and C) The optical coherence tomography images reveal the presence of an intima-media membrane and a large intramural hematoma (yellow asterisks) compromising the true lumen. The white asterisk points at the shadow caused by the guidewire; the circular image in the true lumen corresponds to the intracoronary imaging catheter.
In the fourth place, we have also learned a great deal of information on the management of these patients.1–5 Unlike the ACS due to atherosclerosis where the percutaneous coronary intervention with drug-eluting stent implantation is the treatment of choice, in patients with SCAD the best thing to do is start with conservative treatment1–5,10,11 (Fig. 2). In most cases, clinical signs can stabilize with medical therapy, and disease progression is favorable. Eventually, there is a complete arterial repair. Also, the results of percutaneous coronary intervention are often suboptimal here (leaving residual lesions, unsolved distal dissections) and there is a much higher risk of complications (basically due to the extent of the dissection or the intramural hematoma).10,11 Unfortunately the results of surgical revascularization are not very good either due to the dissected arterial wall friability. That is why most experts recommend sparing revascularization for cases where medical therapy has failed (e.g., persistent or recurrent ischemia).10,11 Drug-eluting stents used in a relatively conservative way with respect to the target segment length are the treatment of choice10,11 (Fig. 2). Vascular fenestration techniques (to communicate with the true lumen and decompress the false lumen), and fully bioresorbable vascular scaffolds are very attractive strategies for selected cases. However, the experience gained on this regard is scarce.
Diagnostic and treatment algorithm of patients with spontaneous coronary artery dissection (SCAD). Upon clinical suspicion, the angiography is the best diagnostic imaging tool available; however, in some cases, intracoronary diagnostic imaging modalities (e.g., optical coherence tomography [OCT]; intravascular ultrasound [IVUS]) may be necessary to achieve a definitive diagnosis. These modalities are especially useful in patients who need to be revascularized. They can also be used (dotted line) in selected cases for a better anatomical diagnosis or with research purposes. Revascularization is indicated in patients with persistent or recurrent ischemia (*). Ideally, drug-eluting stents should be used (although fenestration and other techniques may also be useful) as part of a conservative therapeutic strategy since the entire diseased segment does not need to be treated. However, in most patients, symptoms can stabilize, and the early strategy should be the conservative medical treatment (**). At the follow-up, non-invasive imaging modalities (axial computed tomography scan [CAT], cardiovascular magnetic resonance imaging [CMR]) (***) are useful to rule out the presence of noncoronary heart disease, fibromuscular dysplasia in particular. The CAT scan can also be used for the non-invasive monitoring of coronary abnormalities. In principle, performing other routine invasive procedures at the follow-up would not be justified except for inside the study protocols (straight line).
Finally, regarding the medical therapy, we have learned that the use of anticoagulants is not justified (fibrinolysis would be contraindicated). Also, that antiplatelet therapy should not be as aggressive as it is regarding the treatment of ACS due to atherosclerosis or in the acute phase or in the long run since we don’t have scientific evidence available to support this.1–5 However, the use of beta-blockers is very attractive from the pathophysiological point of view. Actually, some data show that it may actually reduce the rate of recurrences.1–5 Statins and ACEI would be recommended for patients with plasma lipid disorders or ventricular dysfunction, respectively. In patients with SCAD in proximal coronary segments, the computed tomography scan can facilitate the proper non-invasive follow-up The possibility of recurrences should be monitored (∼10% in 3 years), and clinical follow-up should be planned.1–5 Finally, cardiac rehabilitation programs and in-person or online clubs have been welcomed by these patients
In conclusion, we have discussed everything we have learned on SCAD over the last decade. Unfortunately, compared to other cardiovascular diseases, it is evident that, to this date, our knowledge of SCAD is still very limited. Being able to generate the proper scientific evidence based on strategies or controlled therapies compared on a randomized bases is a particularly difficult challenge in the management of this rare condition.3 Therefore, more studies are needed like well-organized multicenter prospective registries at both national and international level.1–3
Conflicts of interestNone reported.
Please cite this article as: Alfonso F, García-Guimaraes M. Disección coronaria espontánea: ¿dónde estamos? Med Intensiva. 2021;45:371–374.





![Diagnostic and treatment algorithm of patients with spontaneous coronary artery dissection (SCAD). Upon clinical suspicion, the angiography is the best diagnostic imaging tool available; however, in some cases, intracoronary diagnostic imaging modalities (e.g., optical coherence tomography [OCT]; intravascular ultrasound [IVUS]) may be necessary to achieve a definitive diagnosis. These modalities are especially useful in patients who need to be revascularized. They can also be used (dotted line) in selected cases for a better anatomical diagnosis or with research purposes. Revascularization is indicated in patients with persistent or recurrent ischemia (*). Ideally, drug-eluting stents should be used (although fenestration and other techniques may also be useful) as part of a conservative therapeutic strategy since the entire diseased segment does not need to be treated. However, in most patients, symptoms can stabilize, and the early strategy should be the conservative medical treatment (**). At the follow-up, non-invasive imaging modalities (axial computed tomography scan [CAT], cardiovascular magnetic resonance imaging [CMR]) (***) are useful to rule out the presence of noncoronary heart disease, fibromuscular dysplasia in particular. The CAT scan can also be used for the non-invasive monitoring of coronary abnormalities. In principle, performing other routine invasive procedures at the follow-up would not be justified except for inside the study protocols (straight line). Diagnostic and treatment algorithm of patients with spontaneous coronary artery dissection (SCAD). Upon clinical suspicion, the angiography is the best diagnostic imaging tool available; however, in some cases, intracoronary diagnostic imaging modalities (e.g., optical coherence tomography [OCT]; intravascular ultrasound [IVUS]) may be necessary to achieve a definitive diagnosis. These modalities are especially useful in patients who need to be revascularized. They can also be used (dotted line) in selected cases for a better anatomical diagnosis or with research purposes. Revascularization is indicated in patients with persistent or recurrent ischemia (*). Ideally, drug-eluting stents should be used (although fenestration and other techniques may also be useful) as part of a conservative therapeutic strategy since the entire diseased segment does not need to be treated. However, in most patients, symptoms can stabilize, and the early strategy should be the conservative medical treatment (**). At the follow-up, non-invasive imaging modalities (axial computed tomography scan [CAT], cardiovascular magnetic resonance imaging [CMR]) (***) are useful to rule out the presence of noncoronary heart disease, fibromuscular dysplasia in particular. The CAT scan can also be used for the non-invasive monitoring of coronary abnormalities. In principle, performing other routine invasive procedures at the follow-up would not be justified except for inside the study protocols (straight line).](https://static.elsevier.es/multimedia/21735727/0000004500000006/v1_202107200621/S2173572721000576/v1_202107200621/en/main.assets/thumbnail/gr2.jpeg?xkr=1dZuESKpnCAWr3yCSGZ24A==)


