Variables predicting optimal timing for tracheostomy decannulation remain unknown. We aimed to determine whether classifying patients into two groups according to their indications for tracheostomy could identify variables associated with time to decannulation.
DesignA prospective, observational cohort study was carried out.
LocationTwo medical–surgical ICUs.
PatientsWe included all patients tracheostomized during ICU stay, excluding patients with do-not-resuscitate orders, tracheostomies for long-term airway control, neuromuscular disease, or neurological damage. Patients were classified into two groups: patients tracheostomized due to prolonged weaning and/or prolonged mechanical ventilation (Group 1), and patients tracheostomized due to low level of consciousness or inability to manage secretions (Group 2).
InterventionsPatients were weaned and decannulated according to established protocols.
Main variablesWe recorded the following variables: time to tracheostomy, forced vital capacity, peak flow, suctioning requirements, Glasgow Coma Score (GCS), characteristics of respiratory secretions, and swallowing function. Statistical analyses included Cox-proportional multivariate analysis with time to decannulation as the dependent variable.
ResultsA total of 227 patients were tracheostomized in the ICUs; of these, 151 were finally included in the study. In the multivariate analysis, time to decannulation in Group 1 was associated with the male gender (HR 1.74 (1.04–2.89), p=0.03), age>60 years (HR 0.58 (0.36–0.91), p=0.02), high suctioning frequency (HR 0.81 (0.67–0.97), p=0.02), low forced vital capacity (HR 0.48 (0.28–0.82), p<0.01), and low peak flow (HR 0.25 (0.14–0.46), p<0.01). In Group 2 time to decannulation was associated to GCS >13 (HR 2.73 (1.51–4.91), p<0.01), high suctioning frequency (HR 0.7 (0.54–0.91), p<0.01), and inadequate swallowing (HR 1.97 (1.11–3.52), p=0.02).
ConclusionVariables associated with longer time to decannulation in ICU-tracheostomized patients differ with the indications for tracheostomy.
No se han podido desarrollar modelos predictores de tiempo de decanulación de pacientes traqueotomizados. El objetivo del estudio fue desarrollar variables asociadas al tiempo empleado en decanular a los pacientes, mediante la clasificación de los pacientes según la indicación de la traqueotomía (TRQ).
DiseñoEstudio de cohortes prospectivo observacional.
ÁmbitoDos UCI médico-quirúrgicas.
PacientesSe incluyeron todos los pacientes traqueotomizados en UCI, excluyendo aquellos con órdenes de no resucitación, TRQ crónicas, enfermos neuromusculares o con daño cerebral. Fueron clasificados en 2 grupos: traqueotomizados por ventilación mecánica o destete prolongado (Grupo 1) y pacientes traqueotomizados por disminución del nivel de conciencia o incapacidad para manejar las secreciones respiratorias (Grupo 2).
IntervencionesSe empleó un protocolo de destete y decanulación.
Variables de interés principalesSe recogieron entre otras las siguientes variables: tiempo hasta decanulación, capacidad vital y flujo espiratorio máximo, necesidades de aspiración, Glasgow Coma Store (GCS), características de las secreciones respiratorias y función deglutoria. Se realizó un análisis multivariable proporcional de Cox, siendo el tiempo hasta decanulación la variable dependiente.
ResultadosDe 227 pacientes traqueotomizados, 151 fueron incluidos en el estudio. El estudio multivariable seleccionó en el grupo 1 las variables: género masculino (HR 1,74 [1,04-2,89], p=0,03), edad >60 años (HR 0,58 [0,36-0,91], p=0,02), requerimiento de aspiraciones elevado (HR 0,81 [0,67-0,97], p=0,02), capacidad vital forzada menor (HR 0,48 [0,28-0,82], p<0,01), y flujo espiratorio pico bajo (HR 0,25 [0,14-0,46], p<0,01); y en el grupo 2 con GCS >13 (HR 2,73 [1,51-4,91], p<0,01), requerimiento de aspiraciones elevado (HR 0,7 [0,54-0,91], p<0,01), y deglución inadecuada (HR 1,97 [1,11-3,52], p=0,02).
Conclusiónlas variables asociadas con el tiempo hasta decanulación en pacientes críticos difieren según la indicación de la traqueotomía.
Increasing importance is being given to decannulation time in tracheostomized patients following recovery from critical illness, since there is growing evidence that tracheostomized patients are at increased risk when managed in conventional hospital wards.1–5 On the other hand, however, postponing patient discharge from the Intensive Care Unit (ICU) is an extremely expensive alternative.6,7
The limited scientific knowledge available in this field is mainly based on epidemiological studies, opinion surveys and observational studies8–13–a situation that has given rise to a broad variety of decannulation practices among the different centers.10,13–15 In this context, clinical protocols focused on decision making at the patient bedside and based on evidence would be very useful for establishing a prognosis and in planning resource utilization in the ICU.
Previous attempts to develop a predictive model have had limited success in predicting the outcome of tracheostomized critical patients,10,16 probably as a consequence of the many variables that affect the final prognosis and the great heterogeneity of tracheostomized patients.17,18 Clinically, tracheostomized patients in the ICU can be divided into two main groups: those who require tracheostomy in the context of mechanical ventilation (MV) or prolonged weaning, and those who require tracheostomy because of incapacity to manage respiratory secretions–including patients with diminished consciousness secondary to brain damage.
Our working hypothesis was that the heterogeneous case-mix represented by these patients can complicate the study of variables that predict delays in decannulation, limiting the development of a predictive model. Our objective was to determine whether the patient classification in these two tracheostomy groups based on the indication of tracheostomy can facilitate the identification of possible variables associated to delays in time to decannulation.
Patients and methodsPatientsOver a period of 14 months (November 2008–December 2009), we evaluated all the patients admitted to two ICUs: a 26-bed, closed clinical–surgical ICU belonging to a tertiary hospital with 700 beds, and an 8-bed general ICU without neurocritical patients belonging to a secondary hospital with 300 beds. The Ethics Committees of both centers approved the study, though informed consent was not requested, since the study was of a purely observational nature.
The study included patients tracheostomized in the context of prolonged mechanical ventilation (MV) (>21 days) or prolonged weaning19 (group 1), and patients tracheostomized due to neurological defects or the incapacity to manage respiratory secretions (group 2). Neurological defects were defined as a motor component on the Glasgow Coma Scale (GCS) of <6 points on the day on which tracheostomy was performed. The patients were considered unable to manage their respiratory secretions when two or more failed extubation procedures were registered because of the retention of secretions, or when the patient was not extubated after tolerating a weaning test because the respiratory secretions had to be aspirated ≥3 times in an hour or ≥8 times in 8h. Patients presenting criteria for inclusion in both groups were assigned to group 1 to the effects of analysis.
The exclusion criteria were a patient age of <18 years, tracheostomy performed prior to admission to the ICU, a motor component of the GCS of <6 at the time of attempted decannulation, tracheostomy indicated for long-term protection of the airway, neuromuscular disease (e.g., lateral amyotrophic sclerosis, Guillain–Barré syndrome), patients with non-resuscitation instructions, or the transfer to chronic care units of patients with a need for partial ventilation support. Patients with early tracheostomy (MV<7 days) were also excluded, because such procedures were only performed in patients with serious brain damage at the time of admission to the ICU.
Weaning and decannulation protocolsDaily patient assessment was carried out, using the following criteria to time weaning19: patients in the recovery phase of the disease giving rise to the need for MV; respiratory criteria (PaO2/fraction of inspired oxygen (FiO2) >150mmHg with positive end-expiration pressure (PEEP)<8cm H2O and arterial pH>7.32); and clinical criteria (absence of ECG evidence of myocardial ischemia, no need for vasoactive drugs or dopamine (≤5μg/kg/min), heart rate<140beats/min, hemoglobin>8g/dl, temperature<38°C, no need for sedation, presence of respiratory stimulus, presence of adequate spontaneous cough reflex, and GCS>8 points–exclusively comprising the ocular and motor components).
The tracheostomized patients were progressively weaned from MV according to a clinical algorithm comprising progressive supportive pressure reduction or T-tube disconnections, according to the decision of the supervising clinician.20 When the patients tolerated at least 12 consecutive hours of disconnection during two successive days they were switched to a continuous T-tube. Weaning failure in turn was defined as the reintroduction of ventilatory support in the 72h following 24h without MV.
Decannulation protocolAfter tolerating 24 consecutive hours of disconnection, the patients were evaluated for possible decannulation. Firstly, an occlusion test21 was performed to discard possible airway obstruction. Briefly, the cannula was replaced by a fenestrated cannula, withdrawing the internal cannula. The tip of the cannula was then occluded for 5min. If the patient showed changes in heart rate, respiratory frequency or arterial pressure suggestive of tracheal stenosis, bronchoscopy was performed.
In the second step of the protocol, evaluation was made of the capacity of the patients to avoid aspirations (see below). Those patients with a normal swallowing test received an oral diet (involving a cannula without cuff inflation) in appropriate cases; in the rest of the cases, an enteral diet was provided through a nasogastric or jejunal tube until definitive disconnection from MV.
The third step in the decannulation protocol included a clinical evaluation by the supervising physician to assess the capacity of the patient to adequately manage the respiratory secretions. The assessment was mainly based on the frequency of the need for suctioning and the characteristics of the respiratory secretions, as explained further below.
The patients were decannulated when they met the following criteria: (1) negative occlusion test, discarding airway obstruction at tracheal level; (2) adequate capacity to manage the respiratory secretions, defined as a need for suctioning ≤2 times every 8h; and (3) low aspiration risk (normal swallowing test).
When the patients failed to meet these criteria, the tracheal cannula was switched to a fenestrated cannula with an internal diameter of ≤7mm and an internal tube. In the case of patients with a low aspiration risk (normal swallowing test), the replaced cannula cuff was, moreover, not inflated.
The tracheal cannula cuff was inflated when suctioning proved necessary with a frequency of <4 times in 8h, and MV disconnection had been maintained during >48h. If plugging was tolerated during 24 consecutive hours, and not removed to suction the respiratory secretions, decannulation was carried out.
Based on previous studies,1,5 the tracheostomized patients remained in the ICU until decannulation or until decannulation was not considered possible (>120 days after definitive disconnection from MV). The patients classified as presenting “expectable in-hospital death” at discharge from the ICU (Sabadell score 3) were excluded from the study and discharged to selected hospital wards after only 10 days of decannulation attempts.
Decannulation failure was defined as the need to intubate or re-cannulate the patients within the 96h following decannulation.
Data collectionThe following information was collected on a prospective basis: patient age, gender, APACHE II score upon admission to the ICU, diagnosis leading to admission, comorbidities, duration of MV, and duration of stay in the ICU and in hospital. In addition, variables related to tracheostomy were recorded: indication, performance time, technique, complications of the procedure and related adverse events (mainly malpositioning and occlusion episodes) during stay both in the ICU and in hospital. In turn, the following variables were documented upon weaning from MV: GCS, forced vital capacity (FVC) and spontaneous peak expiratory flow (PEF), the frequency of aspirations, and volume of the secretions (suctioned and total, obtained by summing spontaneous expectoration) during the previous 8h.
Vital capacity (VC) was measured with a Wright/Haloscale Respirometer® (Ferraris Respiratory Europe, Hertford SG13 7NW, England), and PEF was determined with a Mini-Wright® Peak Flow Meter (Clement Clarke International Ltd., Essex CM20 2TT, England). Both variables were measured with the tracheostomy cuff both inflated and deflated, in order to determine whether plugging affected the capacity of the model used. The clinical variables were recorded in the 8h after complying with the weaning criteria.
Aspiration risk was assessed by means of the swallowing test. Basically, the latter involves the swallowing of 50ml of water with the tracheostomy cuff deflated.22,23 The results of the swallowing test were classified as follows: (1) normal (≤5 swallows in <10s); (2) abnormal (>5 swallows in ≥10s, or clinical evidence of aspiration during the test); or (3) severe dysfunction (spontaneous aspiration of saliva or pharyngeal secretions, contraindicating performance of the test). The measurements were made on a daily basis, until the test results proved normal.
The respiratory secretions were clinically classified as watery, foamy, thick or almost solid.24 Needs for airway care were identified, determining the volume and viscosity of both the expectorated and the suctioned secretions. Viscosity was assessed by measuring the time required to travel the surface of the Muco-Safe® suctioning container (Unomedical A/S, Birkeroed, Denmark). In order to optimize simulation of the clinical scenario, the supervising physician was blinded to the results of these quantitative variables.
Statistical analysisContinuous variables were reported as the mean and standard deviation (SD) in the case of variables with a normal distribution, and as the median and interquartile range (IQR) in the case of a non-normal distribution.
The Student-test was used to compare continuous variables, while the Kruskal–Wallis or Mann–Whitney U-test was applied in the case of samples with <30 individuals. The chi-squared test with Yates correction or the two-tailed Fisher exact test was used to contrast categorical variables.
Kaplan–Meier survival analysis was used to compare time to decannulation in the two groups.
Our working hypothesis was based on previous clinical observations of differences between the two groups in the time from tracheostomy to weaning, and in the time from weaning to decannulation. Accordingly, we constructed a multivariate model for each group, using Cox regression analysis to identify the variables associated to the time interval from weaning to decannulation. The results are expressed as hazard ratios (HR, with the corresponding 95% confidence interval (95%CI)), where HR>1 corresponds to a factor that reduces the time to decannulation.
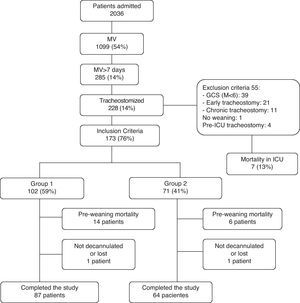
ResultsThe patient flow is shown in Fig. 1. A total of 2036 patients were admitted during the 14 months of the study; 227 of these patients were tracheostomized. A total of 173 (76%) satisfied the inclusion criteria: 102 (59%) subjects, including 12 that met criteria corresponding to both groups, were classified as belonging to group 1 (patients tracheostomized in the context of prolonged MV or weaning), while 71 (41%) patients were assigned to group 2 (patients tracheostomized due to neurological defects or the incapacity to manage respiratory secretions). Lastly, 87 (85%) patients in group 1 and 64 (90%) patients in group 2 were decannulated and completed follow-up. Weaning failed in 5 patients, while the decannulation failure rate was 0%.
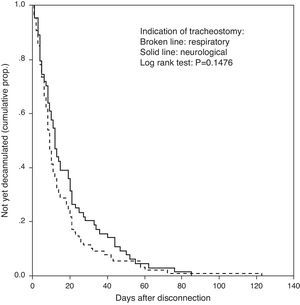
Table 1 shows the clinical characteristics of each group. The difference in time from weaning from MV to decannulation (median [IQR], 9 (5–19) days in group 1 versus 12 (5–24) days in group 2) was not statistically significant (p=0.14). Fig. 2 shows the corresponding Kaplan–Meier curve.
Clinical characteristics of the patients. The data are expressed as the mean±SD, median (interquartile range), or percentage (%).
| Group 1No.=87 | Group 2No.=64 | p | |
| Age, years | 56.4±17.1 | 52.6±18.5 | 0.2 |
| Male gender | 69% | 75% | 0.4 |
| APACHE II score | 19 (14–24) | 18 (15–21) | 0.05 |
| Pre-tracheostomy VAP | 49% | 39% | 0.2 |
| BMI, kg/m2 | 28.1±6 | 27.1±5.8 | 0.03 |
| GCS score | 12 (11–15) | 12 (11–14) | 0.3 |
| Tracheostomy with surgical technique | 35.6% | 31.3% | 0.6 |
| Comorbidity | |||
| Neurological disease | 23% | 43.8% | 0.1 |
| Respiratory disease | 27.6% | 12.5% | |
| COPD | 26.4% | 12.5% | |
| Restrictive pulmonary disease | 1.1% | 0% | |
| Heart disease | 12.6% | 12.5% | |
| Diabetes mellitus | 20.6% | 12.5% | |
| Arterial hypertension | 22.9% | 18.7% | |
| Cancer | 1.1% | 4.7% | |
| Chronic renal failure | 6.9% | 1.5% | |
| Chronic liver disease | 4.6% | 1.5% | |
| Diagnosis upon admission | |||
| Lung injury | 23 (26%) | 12 (19%) | 0.02 |
| Heart disease | 15 (17%) | 1 (1%) | |
| Abdominal injury | 6 (7%) | 1 (1%) | |
| Sepsis (other foci) | 7 (8%) | 2 (3%) | |
| Multiple trauma | 20 (23%) | 31 (48%) | |
| Head injury | 5 (6%) | 30 (47%) | |
| Neurosurgery | 6 (7%) | 15 (23%) | |
| Programmed surgery | 5 (6%) | 0 (0%) | |
| Emergency surgery | 5 (6%) | 2 (3%) | |
| Expiratory function | |||
| FVC ml (inflated cuff) | 715±306 | 676±257 | 0.4 |
| FVC ml (deflated cuff) | 749±333 | 691±283 | 0.2 |
| PEF l/min (inflated cuff) | 104±66 | 109±155 | 0.8 |
| PEF l/min (deflated cuff) | 118±77 | 127±171 | 0.7 |
| Management of respiratory secretions | |||
| Frequency of suctioning in 8h | 1.9±1.3 | 1.8±1.5 | 0.8 |
| Suctioned volume, ml/suction | 2±2.1 | 1.8±1.5 | 0.5 |
| Total secretion volume, ml/8h | 33.2±29.5 | 33±26.7 | 0.9 |
| Total suctioned volume, ml/8h | 5.5±8.1 | 4.6±6.1 | 0.5 |
| Swallowing function | |||
| Normal swallowing test | 48.3% | 45.3% | 0.6 |
| Abnormal swallowing test | 46% | 51.5% | |
| Severe swallowing dysfunction | 5.7% | 3.1% | |
| Viscosity of secretions | |||
| Watery secretions | 69% | 71.9% | 0.3 |
| Thick secretions | 26.4% | 18.8% | |
| Almost solid secretions | 4.6% | 9.4% | |
| Prognosis | |||
| Duration of MV (days) | 33 (24–48) | 15 (10–22) | <0.01 |
| Time to tracheostomy (days) | 21 (15–29) | 11 (8–17) | <0.01 |
| Tracheostomy to weaning (days) | 13 (2–18) | 4 (1–5) | <0.01 |
| Weaning to decannulation (days) | 9 (5–19) | 12 (5–24) | 0.2 |
| Stay in ICU (days) | 46 (31–62) | 25 (17–32) | <0.01 |
| Hospital stay (days) | 74 (55–97) | 46 (34–64) | <0.01 |
APACHE, acute physiology and chronic health evaluation; VAP, ventilator associated pneumonia; FVC, forced vital capacity; COPD, chronic obstructive pulmonary disease; PEF, peak expiratory flow; MV, mechanical ventilation.
Tolerance of tracheostomy plugging was assessed in 42 (48%) patients in group 1 and in 26 (41%) patients in group 2, though only 9 (10%) and 7 (11%), respectively, were decannulated on the basis of this tolerance criterion.
Following the survival analysis, the relevant variables and cutoff points were established as follows: gender, age (60 years), frequency of aspirations during the previous 8h, low FVC measured with the tracheostomy cuff deflated (percentile 33), low PEF measured with the tracheostomy cuff deflated (percentile 33), swallowing function (normal versus abnormal swallowing test), and GCS score (13 points).
Cox regression analysis in turn identified 5 variables associated with the time to decannulation in group 1, and three variables in group 2 (Table 2), though it failed to select any variable when the analysis was applied to the global population (i.e., the sum of both patient groups).
Factors significantly associated to the probability of decannulation per unit time in the multivariate Cox regression analysis.
| Variable | Coefficient | HR (95%CI) | p-Value |
| Group 1 | |||
| Male gender | 0.55 | 1.74 (1.04–2.89) | 0.03 |
| Age (>60 years) | −0.54 | 0.58 (0.36–0.91) | 0.02 |
| Frequency of suctioning in 8h | −0.21 | 0.81 (0.67–0.97) | 0.02 |
| FVC<percentile 33 (cuff deflated) | −0.72 | 0.48 (0.24–0.82) | <0.01 |
| PEF<percentile 33 (cuff deflated) | −1.35 | 0.25 (0.14–0.46) | <0.01 |
| Group 2 | |||
| GCS score (>13 points) | 1.05 | 2.73 (1.51–4.91) | <0.01 |
| Frequency of suctioning in 8h | −0.35 | 0.7 (0.54–0.91) | <0.01 |
| Normal swallowing | 0.68 | 1.97 (1.11–3.52) | 0.02 |
The multivariate regression analysis identified no variable associated to time from weaning to decannulation in the global study population. However, on classifying the patients according to the indication of tracheostomy (i.e., tracheostomy due to persistent respiratory failure or to incapacity to maintain a permeable airway), the multivariate regression analysis detected different variables associated to a longer time from weaning to decannulation in each group.
In addition, the variables associated to the time interval to decannulation differed on comparing them with prognostic variables among tracheostomized patients identified in previous studies.1 In effect, while the characteristics of the respiratory secretions have been related to patient prognosis following discharge from the ICU in other studies,1 this variable did not affect the decannulation process in the ICU in our study.
Some variables not related to the airway or to lung function (patient age, diagnosis leading to admission, previous disease) have repeatedly been related to the decannulation process.1,9,10 In our study, only an association to age and gender was confirmed, but not to either the diagnosis or to previous disease. These variables may influence clinicians in their decision to perform decannulation, though our findings do not confirm such practice. Indeed, none of the abovementioned variables were associated to time to decannulation in group 2, thus suggesting that the decannulation process is very closely related to management of the airway in this subgroup of patients.
Other respiratory parameters such as oxygenation or respiratory frequency10 were not selected by the multivariate regression analysis, probably because they were included in the weaning protocol, i.e., a process different from decannulation.
Our results suggest that patient capacity to manage the respiratory secretions is a complex phenomenon that could be related to other variables in addition to cough reflex, as deduced from a greater total secretion volume (>30ml/8h), a low percentage of suctioning in relation to the total expectoration volume (<20%), a low volume of secretions suctioned in each suctioning maneuver (<2ml), and a lack of correlation between the characteristics of the respiratory secretions and the decannulation process. A possible explanation is interference on the part of the artificial airway, which could complicate patent capacity to manage the secretions, independently of their amount or characteristics. This hypothesis is supported by our data, which reveal greater PEF and FVC on performing the measurements with the tracheostomy cuff deflated compared with measurement with the cuff inflated–thus suggesting that an effective increase in airway diameter could allow increased end-inspiratory lung volume and expiratory function.25 Moreover, the protocol proposed by Ceriana et al.8 includes switching to a cannula with a diameter of ≤6mm, which is even smaller than that in our protocol.
Some of our findings contradict the results presented by other authors. Recently, the epidemiological study published by Stelfox et al.10 found the capacity to tolerate plugging of the cannula to be one of the most important factors cited by clinicians in deciding decannulation. In any case, our data suggest that the capacity to tolerate plugging has low sensitivity and low specificity, since some patents who do not tolerate plugging are successfully decannulated, while in contrast most patients are decannulated without the use of plugging. In addition, the diameter of the cannula could interfere with the tolerance of plugging, and the criteria for unplugging are based on clinical experience and have not been standardized. Our criterion for plugging failure may seem too conservative; some patients who do not tolerate plugging (most with large diameter cannulas) were successfully decannulated, while other patients with inadequate management of the secretions (requiring suctioning 3–5 times every 8h) were able to expectorate effectively once the cannula was unplugged after switching to a smaller diameter.
Our times from weaning from MV to decannulation showed no differences between the two groups, in contrast to the observations of Ary-Jan et al.,18 probably because of the differences in the weaning protocols employed.
Severe swallowing dysfunction was very infrequent in our study. Probably, the prolonged time elapsed from weaning to clinical evaluation allowed the patients to at least partially recover swallowing function. The percentage of patients with moderate swallowing dysfunction in our study was similar to that published by Romero et al.,23 but in contrast to the latter publication, swallowing function failed to reach statistical significance in the multivariate regression analysis of the non-neurological patient subgroup. Some possible explanations for these conflicting data could be the existence of substantial differences in the way swallowing function was measured (in our series the measurements were made with water after deflating the cuff) and in the study populations involved (we included patients with surgical tracheostomies). Indeed, even our nutrition and serial measurements protocol could have avoided aspiration episodes and shortened the time to decannulation.
Study limitationsOur sample size (fewer than 100 patients in each group) could have caused over-adjustments in the statistical model. This phenomenon is even more likely on considering that we recorded no differences before selecting the subgroups. On the other hand, our decannulation protocol is not based on scientific evidence, allowing variations in the time to decannulation, on establishing comparisons with other centers. In a recent epidemiological study, the optimum decannulation failure rate was estimated to be between 2% and 5%.9 Although this percentage is not based on evidence, our own percentage of 0% suggests that the protocol we use is very conservative, and could effectively delay the decannulation of patients who are ready for decannulation. Furthermore, the low weaning failure rate suggests that our weaning protocol is also conservative. As a result, the time from tracheostomy to weaning could be longer in our study than in other publications.18 Lastly, the definition of patient incapacity to manage the respiratory secretions always contains a subjective element, and we chose a very restrictive criterion in order to clearly separate the two patient groups.
Only the patients with a low level of consciousness at the time of tracheostomy and who posteriorly regained consciousness were included in the study, since cooperation was necessary for measuring most of the variables.
In conclusion, the classification of tracheostomized patients according to the indication of tracheostomy is a fundamental step in developing decannulation predictive models.
Conflicts of interestThe authors declare that they have no conflicts of interest.
The authors thank all the patients, and the medical and nursing personnel, for their cooperation in this study.
Please cite this article as: Hernández G, et al. La indicación de la traqueotomía condiciona las variables predictoras del tiempo hasta la decanulación en pacientes críticos. Med Intensiva. 2012;36:531–9.
The study protocol was approved by the Health Department of Castilla la Mancha (SESCAM) (to which Virgen de la Salud Tertiary Hospital is ascribed) and by the Health Department of the Community of Madrid (to which Infanta Sofía University Hospital is ascribed).
This study was presented in abstract form at the 23rd Annual Congress of the ESICM, 12 October 2010, Barcelona (Spain).