Cardiogenic shock is characterized by tissue hypoperfusion due to the inadequate cardiac output to maintain the tissue oxygen demand. Despite some advances in cardiogenic shock management, extremely high mortality is still associated with this clinical syndrome. Its management is based on the immediate stabilization of hemodynamic parameters through medical care and the use of mechanical circulatory supports in specialized centers. This review aims to understand the cardiogenic shock current medical treatment, consisting mainly of inotropic drugs, vasopressors and coronary revascularization. In addition, we highlight the relevance of applying measures to other organ levels based on the optimization of mechanical ventilation and the appropriate initiation of renal replacement therapy.
El shock cardiogénico es un estado de hipoperfusión tisular provocado por la incapacidad del corazón para mantener un gasto cardíaco adecuado a la demanda tisular de oxígeno. A pesar de los avances en el manejo del shock cardiogénico, la mortalidad asociada a este síndrome continúa siendo elevada. Su tratamiento se basa en la estabilización inmediata de los parámetros hemodinámicos mediante la atención médica y el empleo de soportes circulatorios mecánicos en centros especializados. Esta revisión tiene como objetivo conocer el tratamiento médico actual del shock cardiogénico, compuesto principalmente por fármacos inotrópicos, vasopresores y la revascularización coronaria. Además, se detalla la importancia de la aplicación de medidas a otros niveles, basadas en la optimización de la ventilación mecánica y el inicio adecuado de las técnicas de depuración renal.
Cardiogenic shock (CS) as the most severe stage of heart failure is associated with a high morbidity and mortality rate. A deep understanding of its pathophysiology, as well as therapeutic options, are crucial to prevent the development of multiple organ failure. Early, comprehensive, and multidisciplinary care is essential to prevent clinical deterioration and maintain an optimal situation that makes the patient eligible for other therapeutic options related to their condition. Intensive care inits enable the application of all these measures in a compregensive and early manner, improving not only mortality but also reducing the associated morbidity.
Coronary revascularizationEmergency coronary angiography should be performed in all patients with suspected CS due to acute myocardial infarction (AMI) to evaluate coronary anatomy and treat the culprit lesion.1,2 In patients with CS of non-ischemic or unknown etiology, it may be considered urgently or electively.
Early percutaneous coronary revascular (PCI) of the culprit artery is the cornerstone of treatment and the only therapy that has demonstrated a significant reduction in mortality. In the SHOCK clinical trial, compared to an initial medical strategy, immediate revascularization reduce the 6-month (50.3% vs 63.1%, 95%CI, 23.2%–0.9%, P = .027)3 and 1-year mortality rates (53.3% vs 66.4%, 95%CI, 24.1%–2.2%, P < .03).4 Due to the widespread use of early revascularization, the mortality of AMI-related CS has dropped from 70% to 80% in historical cohorts down to 40%–50% in current series and registries.
Delayed revascularization in CS is associated with lower survival rates.5 A recent analysis of 12,675 patients (FITT-STEMI study) emphasizes the strong impact delayed PCIs have on mortality, especially in patients with CS to the point that every 10-minute delay in revascularization within 60−180 min from first medical contact resulted in 3.3 additional deaths per 100 patients treated with PCI.
The presence of multivessel coronary artery disease is a common finding (70% up to 80%) in patients with AMI and CS and is associated with higher morbidity and mortality. The CULPRIT-SHOCK trial6 demonstrated that in patients with CS and multivessel disease, initial revascularization of only the culprit vessel results in lower mortality rates (45.9%) and fewer complications than multivessel revascularization (55.4%). However, in a recent study,7 the multivessel percutaneous approach under mechanical circulatory support (Impella®) had similar results to the strategy of treating the culprit vessel only.
Regarding other types of revascularization, data on the utility of fibrinolysis in the presence of CS are scarce since these patients are usually excluded from the studies, and given this therapy has limited efficacy. Currently, emergent cysurgical revascularization is rare (<4%) and is reserved for patients in whom PCI is not feasible or has failed and due to mechanical complications of AMI.8
Radial access is the recommended approach when performing PCIs as it reduces cardiovascular complications, major bleeding, and mortality compared with femoral access.9 If not possible, femoral access with the use of techniques that reduce local complications such as ultrasound, micropuncture, initial and final angiography, and specific hemostasis protocols is advised.
Antiplatelet and anticoagulant therapyEarly antiplatelet and anticoagulant therapy is recommended in all cases of AMI-related CS. Patients should receive aspirin and heparin at the time of clinical presentation. Sodium heparin is recommended over low molecular weight heparin due to possible decreased subcutaneous absorption. Retrospective studies have shown lower efficacy of clopidogrel, while ticagrelor and prasugrel have a faster and more predictable effect on platelet inhibition. IV antiplatelets such as cangrelor and antiIIb-IIIa might be useful due to their higher platelet activity, but there are no conclusive data on this regard. The potent antiplatelet effect of these IV drugs must always be weighed against the risk of bleeding, especially if femoral access has been used or mechanical devices are employed.
Antiarrhythmic therapyThe incidence of ventricular and supraventricular arrhythmias is very high in CS. There are no recommendations on their specific management, although restoring sinus rhythm or controlling heart rate when this is not possible usually results in hemodynamic improvement.
In cases of atrial fibrillation with hemodynamic instability, sinus rhythm should be restored as soon as possible with electrical cardioversion. If accompanied by a stable situation, rhythm and rate control strategies are acceptable and should be individualized. In this case, amiodarone is the drug of choice for these critical patients.10
The occurrence of recurrent ventricular arrhythmias or arrhythmic storm is a highly impactful situation. Like any other type of arrhythmia, if there is poor hemodynamic tolerance, immediate electrical cardioversion is advised. In the case of stability, amiodarone is the first-line drug in the absence of contraindications, with other antiarrhythmics such as lidocaine or procainamide as second-line options. In more complex scenarios of refractory arrhythmic storm, adequate analgesia, or even deep sedation and intubation, are recommended.
There are no specific recommendations either for patients in CS who experience high-grade conduction disorders. First-line drugs such as atropine or beta-adrenergics (isoproterenol, dopamine, dobutamine) seem reasonable initially. In cases of third-degree AV block refractory to medical treatment, the implantation of a temporary transvenous pacemaker is indicated. Intraventricular conduction disorders, especially left bundle branch block, are also prevalent. The option of cardiac resynchronization through an electro-catheter used in the coronary sinus is a suggestive option, though with insufficient evidence to be widely recommended.10
Management of fluid therapy and diureticsThe first step in the management of CS is optimizing volemic status, along with the restoration of mean arterial pressure (MAP) and organ perfusion.
Patients with CS without signs of congestion may initially benefit from what is called a “fluid challenge” (e.g., 200−250 mL of crystalloids within 15–30 min), unless there are signs of fluid overload, as it can improve systemic pressure in some responsive patients.6,11
On the contrary, patients with congestion and overload have higher mortality rates, and reducing intra- and extravascular volume should be a therapeutic goal. Once MAP has been optimized, treatment with high-dose furosemide is usually indicated. Thiazides can have a synergistic effect when added to loop diuretics. Non-responders and high-risk patients may benefit from renal replacement therapies. The use of ultrafiltration has not shown clear superiority over an initial approach with diuretics, although early hemofiltration has been associated with better outcomes in terms of mortality in patients with postcardiotomy shock.12
Pharmacological managementVasopressor and inotropic therapyThe use of vasopressor and inotropic drugs is recommended in the initial phase of hemodynamic stabilization to prevent ischemia, restore cellular metabolism, and avoid circulatory collapse whenever possible. In many cases, there is not only an issue of inotropism and cardiac pump failure but also severe tissue ischemia and a vasoplegic syndrome as part of the systemic inflammatory response.13,14
Current guidelines recommend the use of vasopressors to increase blood pressure and perfusion to vital organs (level of recommendation IIa), and the use of inotropics to increase cardiac output, blood pressure, and peripheral perfusion (level of recommendation IIb). The choice of inotropic or vasopressor drug is generally empirical and should be guided by the possible hemodynamic effects of each drug.6 Ideally, the goal is to achieve better tissue perfusion without a significant increase in cardiac workload.15 However, the requirements for vasoactive medication are independently associated with short-term mortality, and there is a dose-dependent relationship: the higher the dose of vasoactive drugs, the greater the risk of death. As a rule of thumb, vasoactive drugs should be used at the lowest possible dose and for the shortest possible time.11,16
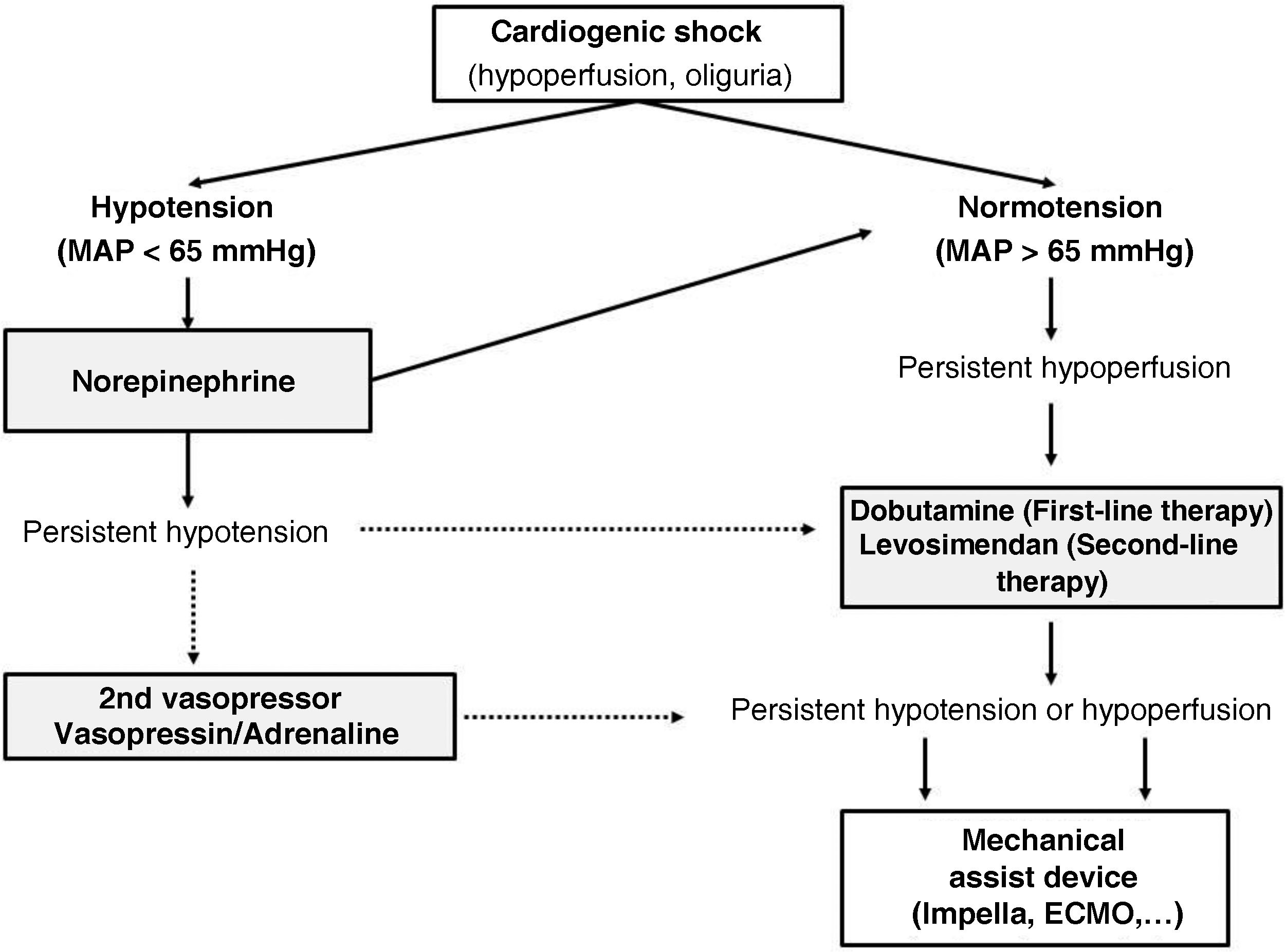
Vasopressor drugsDespite limited evidence available, vasopressor drug therapy is the standard initial hemodynamic support for patients with CS who present with hypotension. It is widely advised to maintain a MAP ≥ 65 and/or a systolic pressure ≥ 90 mmHg, based on extrapolation from other forms of shock,1 and assuming that a MAP < 65 mmHg is associated with worse clinical outcomes. In this context, norepinephrine is the first-line vasopressor drug of choice due to its more favorable safety profile (lower arrhythmogenicity), particularly compared to dopamine or epinephrine.11
In the SOAP-II study,17 a total of 1679 patients (280 with CS) were included, and the effects of norepinephrine vs dopamine as first-line treatment were compared. Dopamine was associated with a higher rate of arrhythmic events, as well as a higher 28-day mortality rate. In the OPTIMA-CC,18 a small study with patients with post-AMI CS, norepinephrine also showed a more favorable profile than epinephrine. Patients treated with epinephrine exhibited more tachycardia and myocardial oxygen consumption, more lactic acidosis, and a higher rate of refractory shock. Therefore, its use as a first-line vasoactive agent to treat CS has been replaced by norepinephrine.6,11
Epinephrine might have a second or third-line role when initial blood pressure targets are not met. It is used as a bolus vasopressor in peri-arrest situations, but its continuous and prolonged infusion should be avoided as numerous studies show an unfavorable risk-benefit ratio.19,20
Phenylephrine is a pure vasoconstrictor with very limited utility in CS.13 It may be considered in very specific situations, such as when excess inotropism is detrimental (tachycardiomyopathy or ventricular outflow tract obstruction).
Vasopressin has become a second-line agent to treat post-cardiotomy septic shock and vasoplegic shock, with scant current evidence in traditional CS. It may have a role in cases of refractory vasodilatory shock when added to norepinephrine and in cases of right ventricular failure, as it does not cause pulmonary vasoconstriction. At high doses, it causes intense systemic vasoconstriction and cutaneous necrosis.1,13
Inotropic drugsIn normotensive patients or those whose blood pressure levels have been normalized with norepinephrine but who still show signs of low cardiac output, it is reasonable to consider the administration of an inotropic drug.21
Dobutamine is the most reasonable first-line inotropic drug due to its short course of action. It is often used simultaneously with norepinephrine to improve perfusion pressure and cardiac contractility.6,13,14,22 This combined therapeutic strategy of norepinephrine plus dobutamine has been compared to monotherapy with epinephrine, resulting in more adverse effects (tachyarrhythmias and hypoperfusion) with the latter drug.17
As an alternative to dobutamine, milrinone, a phosphodiesterase-3 inhibitor, emerges. It has been associated with a high rate of arrhythmias and a greater vasodilatory and hypotensive effect.23 The recent DOREMI study24 compared dobutamine and milrinone as first-line inotropic agents, finding no differences between the two drugs regarding survival, efficacy, or safety. Although both drugs cause hypotension, this effect is more intense and prolonged with milrinone, especially in cases of renal dysfunction. Due to its pulmonary vasodilatory action, milrinone can be used in cases of right-sided CS.
In recent years, levosimendan has emerged as an alternative as first-line therapy CS or as bailout therapy with failed dobutamine therapy.25 Its mechanism of action, independent of intracellular calcium concentration, makes it an attractive drug due to its theoretically lower oxygen consumption and arrhythmogenic potential. With a very long half-life thanks to its active metabolite (OR-1896), it can improve inotropism for 7–9 days after the infusion ends. It has been compared to dobutamine or placebo, showing a beneficial role for levosimendan, although only in patients with SCAI stage A–B, and its utility in more severe cases of CS is unknown to this date.17 It is an interesting drug in adrenergic cardiomyopathy or in weaning from VA-ECMO.19
Except for levosimendan, the other inotropic agents increase intracellular calcium, myocardial oxygen consumption, and the risk of malignant arrhythmias. There is insufficient evidence to favor one over the other, but it appears that dopamine increases mortality, and levosimendan may improve survival. In any case, high doses and/or prolonged use of inotropic drugs should be avoided, and if this occurs, early implantation of mechanical circulatory support should be considered. Milrinone and levosimendan, having a non-adrenergic mode of action, may be more useful than dobutamine in patients on beta-blockers or in beta-blocker intoxication.12
Certain considerations should be made in cases of CS where catecholaminergic drugs can exacerbate the situation. Takotsubo syndrome or stress cardiomyopathy is associated with an increase in circulating catecholamines in susceptible patients. Although there are no specific clinical trials, management includes the cessation of sympathomimetic drugs, short-acting beta-blockers, and the use of levosimendan as the inotropic drug of choice. Similarly, in hypertrophic obstructive cardiomyopathy, catecholamines should be avoided. Maintaining adequate volemia, short-acting beta-blockers, and the use of vasopressor drugs without inotropic effects, such as phenylephrine or vasopressin, are the therapeutic strategy that should be used in these cases.19
In conclusion, we can state that, in the management of CS, vasoactive therapy has barely changed in the last decade, leaving norepinephrine and dobutamine as the vasopressor and inotropic drugs of choice, respectively (Fig. 1). Levosimendan may be an attractive alternative, especially in early stages of shock, beta-blocker intoxication, stress cardiomyopathy, or VA-ECMO weaning. Milrinone can be reserved for cases of right ventricular failure. Most authors recommend using vasopressin or epinephrine if a second vasopressor drug is needed alongside norepinephrine.13
Other drugsLow doses of cardioprotective drugs are beneficial in terms of morbidity and mortality in patients with reduced ejection fraction. However, the best time to start this therapy once the shock situation has passed is still to be elucidated and must be individualized. Therefore, the rule of thumb would be to start slow, go slow. Beta-blockers and other drugs that cause hypotension, such as ACE inhibitors or ARBs, should be administered as tolerated when the hypoperfusion state is resolved, and vasopressor drugs are no longer required.1 Similarly, we will proceed with the new SGLT2 inhibitors. Although some studies suggest their early use after an AMI,19 their benefit in the management of CS is not well established, and they cause hypotension, so they should be initiated once catecholamines are withdrawn.
Temperature control and management after cardiac arrest and CSCentral temperature in healthy individuals is regulated in the hypothalamus.26 Elevated temperature can lead to brain injury by increasing metabolic demand, free radical damage, cerebral edema, and seizures. In hyperthermia, the body's thermoregulatory capacity is overwhelmed.27 Fever is a hypothalamic adjustment in response to infection or inflammation; it is unknown whether it contributes to poor neurological outcomes or is merely a marker of severe brain injury. About 46% of patients will experience hyperthermia or fever within the first 2–3 days after a cardiac arrest (CA),28 which is associated with worse outcomes.29
The beneficial effects of hypothermia in post-CA management include reducing metabolism, allowing organs to tolerate longer periods of ischemia without irreversible damage; decreasing reperfusion injury, especially neurologically induced damage by inflammatory mediators released after blood flow is restored; and mitigating blood-brain barrier dysfunction.30 In 2002, with the publication of two clinical trials in the New England Journal of Medicine (HACA31 and Bernard et al.32) showing the benefit of hypothermia, the results led to the adoption of hypothermia over the prevention of hyperthermia. Subsequent studies, such as the TTM33 (Targeted temperature management) and the FROST-1,34 demonstrated that a lower temperature target (33 °C) did not improve mortality or neurological prognosis vs a target temperature of 36 °C.
In 2021, the European Resuscitation Council (ERC) and the European Society of Intensive Care Medicine (ESICM)35 recommended selecting and maintaining a constant target temperature between 32 °C and 36 °C for patients who remain in a coma or unresponsive after the return of spontaneous circulation (ROSC), in reversed out-of-hospital CA with a shockable rhythm, and with lower evidence levels in in-hospital CA and those with non-shockable rhythms.35
Controlled cooling methods (such as percutaneous, or endovascular) are preferred over conventional uncontrolled methods (cold saline infusion, ice/water blankets…), which are more useful for achieving a faster cooling rate but do not maintain a constant temperature.
Hypothermia in post-CA care is performed in 4 phases: induction, maintenance, rewarming, and normothermia. Cooling should be initiated as soon as possible after ROSC. Although its duration is unknown, better outcomes are observed if it is prolonged beyond 24 h. Shivering, which reduces the cooling rate, can be treated with adequate sedation, magnesium sulfate, and/or neuromuscular relaxants. Afterwards, rewarming should be performed in a controlled manner (0.1 °C–0.5 °C/h).36 Strict normothermia control (36.5 °C–37 °C) for, at least, an additional 24 h prevents rebound fever.
Possible complications of thermal control include coagulopathy, increased infections due to immune dysfunction associated with post-CA inflammatory syndrome, metabolic acidosis due to secondary tissue hypoperfusion, and reduced cardiac output along with electrolyte disturbances (hypokalemia and hypomagnesemia), resulting in arrhythmias and electrocardiographic changes (prolonged PR and QTc intervals, and QRS widening).
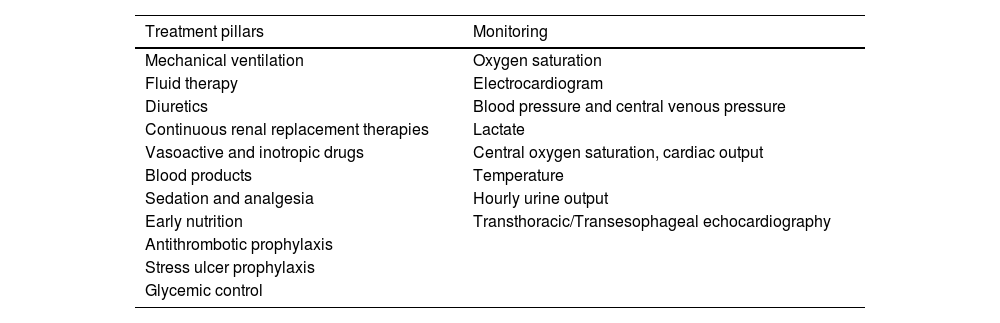
General management at the ICU settingThe general management of patients with CS must be comprehensive and multidisciplinary, coordinated by the intensivist who is responsible for the patient and knowledgeable about the essential treatment and monitoring pillars necessary for proper management (Table 1).
Supportive treatment scheme for multiple organ dysfunction in cardiogenic shock.
| Treatment pillars | Monitoring |
|---|---|
| Mechanical ventilation | Oxygen saturation |
| Fluid therapy | Electrocardiogram |
| Diuretics | Blood pressure and central venous pressure |
| Continuous renal replacement therapies | Lactate |
| Vasoactive and inotropic drugs | Central oxygen saturation, cardiac output |
| Blood products | Temperature |
| Sedation and analgesia | Hourly urine output |
| Early nutrition | Transthoracic/Transesophageal echocardiography |
| Antithrombotic prophylaxis | |
| Stress ulcer prophylaxis | |
| Glycemic control |
CS is often complicated by respiratory failure, and more than 50% of patients will require ventilatory support.37 Cardiogenic pulmonary edema causes an excess of interstitial and alveolar fluids, impairing ventilation, increasing respiratory effort, and ultimately causing hypoxemia and hypercapnia. Current guidelines recommend early intubation and ventilation38 with protective ventilation strategies (TV 6−8 mL/kg).
The hemodynamic effects of mechanical ventilation (MV) can be grouped into 3 relevant concepts:
- 1
Ventilatory support reduces respiratory effort and metabolic demand.
- 2
Changes to lung volume alter autonomic tone and pulmonary vascular resistance.
- 3
MV with positive pressure increases intrathoracic pressure, decreasing venous return.39 Extubation will cause an increase in venous return and sympathetic hyperactivity, suggesting the use of non-invasive ventilation post-extubation to minimize these changes.
Non-invasive MV is the cornerstone of the management of cardiogenic pulmonary edema; however, its role in the management of CS is questionable due to hemodynamic instability and impaired consciousness.
Sedation and analgesiaSedation in the critical care setting is currently based on light sedation protocols; using the minimum necessary dose for adequate control of pain, delirium, and agitation, while adapting to the patient's clinical and hemodynamic characteristics.
The first-line therapy for pain control is opioids. Despite causing hypotension and bradycardia due to direct vasodilation, preload reduction, and modulation of the autonomic nervous system, they do not affect cardiac output, contractility, and decrease myocardial oxygen consumption.40
For sedation, a review of clinical trials showed that benzodiazepines, propofol, and dexmedetomidine share the same sedation efficacy profile without mortality differences. While non-benzodiazepine agents shorten MV time and cause less delirium,41 their use is controversial in cardiac patients due to their hemodynamic effects and potential reduction of cardiac output.42 Propofol leads to hypotension due to direct vasodilation, with sympatholytic effects and bradycardia through muscarinic receptor activation. Although dexmedetomidine is an attractive option inducing light sedation without respiratory suppression, its side effects (hypotension, bradycardia) can be potentially life-threatening in these cases.
Nutritional supportThe prevalence of caloric protein malnutrition in CS is high and is associated with increased mortality and long hospitalizations. In CS, the intestinal mucosa suffers from an impaired blood perfusion, a reduction in splanchnic flow causing perfusion deficits, ischemia, and accumulation of toxic metabolites that increase intestinal permeability and bacterial translocation.
Enteral nutrition (EN) improves splanchnic perfusion and maintains the integrity of the intestinal mucosa, preventing bacterial overgrowth and controlling systemic inflammation.43 In CS, strategies are controversial, but early EN (24−48 h of admission) is currently recommended.44 Its administration will be delayed if hemodynamic and tissue perfusion targets are not achieved or if uncontrolled shock persists.
Patients with CS on vasoactive or mechanical support can be adequately nourished,45 although there is uncertainty surrounding the nutrition of patients on ECMO. Its use usually involves sedation and relaxation, which can affect the intestinal function. However, several recent studies have confirmed that early EN improves prognosis.46,47
In the presence of GI intolerance or mesenteric ischemia, if enteral feeding is not possible, or if the caloric-protein requirement for the patient is not met, parenteral nutrition will be used, either total or complementary.
Management of renal dysfunctionAcute kidney injury (AKI) is a common complication in CS (20% up to 35% of patients).48 AKI is associated with a worse prognosis and higher mortality rates. Early use of renal replacement therapies, management of comorbidities, and fluid administration help improve outcomes.
Cardiorenal syndrome is based on the pathophysiological interaction between the heart and the kidneys. Type 1 consists of abrupt kidney injury and/or dysfunction in the context of acute worsening of cardiac function.49 Hemodynamic factors such as renal hypoperfusion leading to oliguria50 tangle up with non-hemodynamic factors such as inflammation, systemic and venous congestion, right ventricular failure, and contrast-induced nephropathy. The timing of AKI onset can vary: in some cases, cardiac and renal dysfunction are present at admission, while in others, AKI develops during hospitalization.51 These can represent the natural progression of the disease, and also be a complication associated with the medical intervention.
Strict monitoring of renal function and diuresis is advised. The introduction of new biomarkers of kidney injury (neutrophil gelatinase-associated lipocalin [NGAL]) could play a key role here. Its increase in plasma occurs before changes in classical parameters and is less sensitive to hemodynamic changes.52
Diuretics, vasodilators, inotropes, and avoiding nephrotoxins are the pillars of treatment. Continuous renal replacement therapies have been evaluated as a specific therapy. The optimal timing for initiation or discontinuation, duration, and dose of therapy is still a controversial topic. Overall, initiation depends on the occurrence of potentially life-threatening alterations in fluid, electrolyte, and acid-base balance.53
Early mobilizationEarly mobilization of patients in CS is challenging, and evidence is scarce on this regard. Bed rest contributes to multiple short- and long-term complications54,55 (ICU-acquired weakness, neuromuscular weakness, reduced quality of life, hospital readmission, and death). The evidence on the benefit of early mobilization in the ICU is, at this point, inconclusive; it has been suggested to shorten the length of stay and improve physical function at discharge.56 However, there are unresolved controversies on this regard: absolute contraindications for mobilization include active myocardial ischemia, hemodynamic instability, worsening pulmonary congestion, or uncontrolled bleeding.57 Furthermore, the use of mechanical circulatory support devices poses a challenge for early mobilization due to the risk of displacement or malfunction of these devices.58
Other preventive managementDelirium preventionUnlike delirium in ICU patients for different reasons, studies of patients undergoing heart surgery have demonstrated that age, preoperative cognitive impairment, depression, cerebrovascular and peripheral disease, smoking, atrial fibrillation, and renal dysfunction are associated with a higher risk of postoperative delirium.59 A sub-analysis of the CardShock study confirmed that 68% of patients had an altered mental state, and that the 90-day mortality rate was significantly higher in these cases.60
Characteristics associated with a higher risk of delirium include old age, a history of cognitive impairment/delirium in previous hospitalizations, previous heart failure, polypharmacy, a history of drug and alcohol abuse, CA, the use of mechanical circulatory support (MCS) devices, and MV.61
Preventing delirium requires a systematic multidisciplinary approach based on pain assessment and treatment; awakening and spontaneous breathing; appropriate selection of analgesia and sedation; monitoring and management of delirium; early mobility and exercise; and family involvement, avoiding the routine administration of antipsychotics.62
Infection preventionThe most severe and common infections in ICU patients are those related to instrumentation. Proper management and treatment—the cornerstone of critical patient care—are associated with better prognosis and survival. Currently, the role of intensivists is crucial with their participation in antibiotic stewardship programs (PROA). Understanding the microbial resistance map in both the hospital and the ICU setting, as well as participating in infection prevention programs (Zero projects), is fundamental.
Respiratory infections, catheter-related bloodstream infections (CRBSI), and urinary tract infections (UTI) are notable for their incidence rate and severity. The main strategies for preventing these infections include:
- -
Proper hygiene measures by health care personnel
- -
Knowledge of epidemiology and patient follow-up
- -
Implementation of a correct antibiotic policy
- -
Identification of risk factors in patients
- -
Patient isolation to prevent cross-colonization
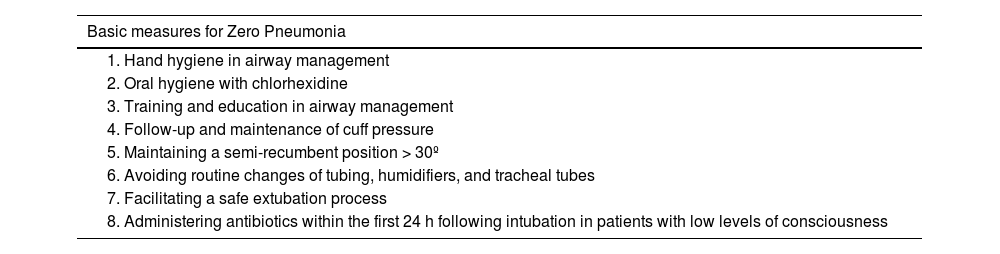
Respiratory tract infections, especially ventilator-associated pneumonia (VAP), are the most common ICU-acquired infections of all. Although invasive mechanical ventilatory support is its trigger, it is estimated that up to one-third of patients who have experienced a cardiac arrest and bronchoaspiration will later develop VAP.63 The implementation of the Zero Pneumonia program64 through preventive measures at the ICU setting has reduced the incidence of VAP. Table 2 describes the basic mandatory measures.
Basic measures for Zero Pneumonia.
| Basic measures for Zero Pneumonia |
|---|
| 1. Hand hygiene in airway management |
| 2. Oral hygiene with chlorhexidine |
| 3. Training and education in airway management |
| 4. Follow-up and maintenance of cuff pressure |
| 5. Maintaining a semi-recumbent position > 30º |
| 6. Avoiding routine changes of tubing, humidifiers, and tracheal tubes |
| 7. Facilitating a safe extubation process |
| 8. Administering antibiotics within the first 24 h following intubation in patients with low levels of consciousness |
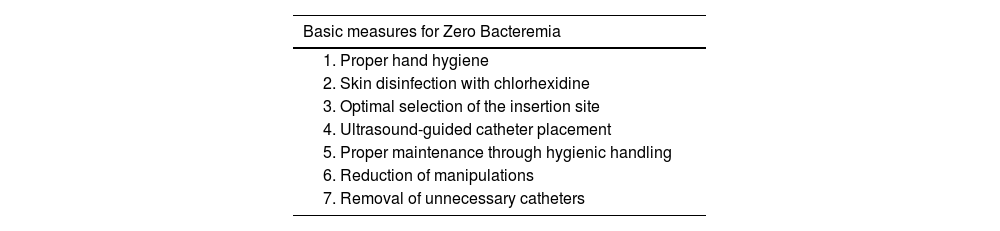
Bloodstream infections resulting from the use of venous and arterial catheters or mechanical circulatory support devices are associated with longer hospitalizations and higher mortality rates. Risk factors for these infections include those related to the host (chronic disease, immunodeficiency, malnutrition, and age) and those related to the catheter (duration and type of catheter, care at the insertion site).65 The Zero Bacteremia program (Table 3), with standardized measures associated with catheter insertion and maintenance, has proven effective in preventing these infections.66
Basic measures for Zero Bacteremia.
| Basic measures for Zero Bacteremia |
|---|
| 1. Proper hand hygiene |
| 2. Skin disinfection with chlorhexidine |
| 3. Optimal selection of the insertion site |
| 4. Ultrasound-guided catheter placement |
| 5. Proper maintenance through hygienic handling |
| 6. Reduction of manipulations |
| 7. Removal of unnecessary catheters |
Finally, UTIs extend hospital stays and increase health care spending. Urinary catheterization is the most important factor associated with UTIs, but other factors include the duration of catheterization, female sex, advanced age, diabetes mellitus, bacterial colonization of the drainage bag, and errors made while handling the catheter.67 The implementation of ITU-Zero measures, based on proper insertion and maintenance of the urinary catheter, with daily evaluations for catheter removal, has shown positive results.
In conclusion, although data on CS patients are limited, the implementation of therapeutic strategies aimed at minimizing infections has shown very positive results.
Palliative careDespite advances made, CS continues to have high morbidity and mortality rates; the incorporation of palliative care in cardiovascular critical care areas is rate. The implementation of educational programs is insufficient and requires greater awareness and improvement in the perception that ICU management and palliative care are not mutually exclusive. It is of paramount importance to understand the benefits of palliative interventions in disease management, prognosis understanding, and end-of-life decision-making.68
ConclusionsThe management of CS, as it happens with its pathophysiology, is very complex and requires a cross-sectional and multidisciplinary approach, based not only on early diagnosis and etiological treatment, but also on the prevention and treatment of infections, renal failure, delirium prevention, and other complications, to avoid developing dysfunction and multiple organ failure. The role of the intensivist as the responsible and knowledgeable figure of this condition, and of the ICU setting as the place to perform techniques and treatment, is crucial to reduce the high morbidity and mortality rates reported.
FundingNone declared.
Conflicts of interestNone declared.