The use of sedatives in Intensive Care Units (ICU) is essential for relieving anxiety and stress in mechanically ventilated patients, and it is related to clinical outcomes, duration of mechanical ventilation, and length of stay in the ICU. Inhaled sedatives offer benefits such as faster awakening and extubation, decreased total opioid and neuromuscular blocking agents (NMB) doses, as well as bronchodilator, anticonvulsant, and cardiopulmonary and neurological protective effects. Inhaled sedation is administered using a specific vaporizer. Isoflurane is the recommended agent due to its efficacy and safety profile. Inhaled sedation is recommended for moderate and deep sedation, prolonged sedation, difficult sedation, patients with acute respiratory distress syndrome (ARDS), status asthmaticus, and super-refractory status epilepticus. By offering these significant advantages, the use of inhaled sedatives allows for a personalized and controlled approach to optimize sedation in the ICU.
El uso de sedantes en las Unidades de Cuidados Intensivos (UCI) es esencial para aliviar la ansiedad y el estrés en pacientes con ventilación mecánica (VM), y está relacionado con el resultado clínico, la duración de la VM y la estancia del paciente en UCI. Los sedantes inhalados ofrecen beneficios como un despertar y extubación más rápidos, disminución de la dosis total de opioides y de bloqueantes neuromusculares (BNM), además de tener efectos broncodilatadores, anticonvulsivantes y protectores cardiopulmonares y neurológicos. La sedación inhalada se administra mediante un vaporizador específico. El isoflurano es el agente recomendado debido a su perfil de eficacia y de seguridad. La sedación inhalada se recomienda en la sedación moderada y profunda, en la sedación prolongada, en la sedación difícil, en pacientes con síndrome de distrés respiratorio agudo (SDRA) estatus asmático y estatus epiléptico superrefractario. Al ofrecer estas ventajas significativas, el uso de sedantes inhalados permite un enfoque personalizado y controlado para optimizar la sedación en UCI.
Sedation plays a fundamental role in the management of critically ill patients admitted to the Intensive Care Unit (ICU), reducing anxiety, facilitating the adaptation to mechanical ventilation (MV), and preventing complications related to agitation.1 However, the intravenous (i.v.) sedatives used in the ICU, such as benzodiazepines, propofol, ketamin and dexmedetomidine,have unpredictable pharmacological characteristics in the critical patient and are not without risks.
Benzodiazepines, especially midazolam, can produce adverse effects when used for prolonged periods, such as drug accumulation, delayed awakening, prolongation of MV, and the development of tachyphylaxis and withdrawal symptoms following discontinuation of the medication.2 Moreover, they constitute a risk factor for the appearance of delirium.3 A debate in favor of and against the use of midazolam during MV in patients admitted to the ICU has recently been published.4,5 Likewise, the use of extracorporeal support measures such as continuous renal replacement techniques and extracorporeal membrane oxygenation (ECMO), affects the pharmacokinetics of intravenous sedatives.6,7
The use of propofol can produce bradycardia, hypotension and hypertriglyceridemia. Furthermore, at high doses or during prolonged infusions, this drug can give rise to severe metabolic acidosis associated with potentially fatal cardiogenic shock known as propofol-related infusion syndrome (PRIS). An infusion lasting over 48 h at a dose of ≥83 µg/kg/min has been associated with the development of PRIS. A retrospective analysis by the same authors revealed a prevalence of PRIS of 2.9% and an associated mortality rate of 36.8%.8
Ketamine produces catecholamine release and can lead to a rise in heart rate and arterial pressure. It may also cause dissociation between the limbic system and the thalamus-neocortical tract, resulting in dysphoria, hallucinations, disorientation, vivid dreams and sensory and/or perceptual illusions.9
On the other hand, dexmedetomidine, which can also cause bradycardia and hypotension, is only useful for mild sedationand is not adequate for moderate or deep sedation.10
The Spanish Agency for Medicinal Products and Medical Devices (AEMPS) has recently approved the use of isoflurane for moderate or deep sedation in adults admitted to the ICU who require MV.
The Sedation, Analgesia and Delirium Working Group (GTSAD) of the Spanish Society of Intensive and Critical Care Medicine and Coronary Units (SEMICYUC) has developed the present document offering a review based on the available scientific evidence. It highlights the benefits, indications, adverse effects, dosing and monitoring strategies and management of the technique, to provide a practical tool for the effective incorporation of inhaled sedation in the ICU.
Characteristics of the inhaled agentsWhat inhaled agents are available for the sedation of adult patients admitted to the ICU?The inhaled sedatives currently available in our setting are isoflurane and sevoflurane. These drugs depress neurotransmission pathways at different levels. Their sedative action is mainly produced at a pre-synaptic level in the central nervous system, stimulating GABA and glycine inhibitory receptors, and at a post-synaptic level, inhibiting excitatory receptors such as those of serotonin (5-HT3)), acetylcholine and glutamate. In addition, they exert a direct analgesic effect at the spinal cord level.11
What are the main characteristics of isoflurane and sevoflurane?IsofluraneIsoflurane has a time to onset of action of 7 min, which facilitates gradual dose adjustment to reach the desired level of sedation. Approximately 0.2% of the administered drug is metabolized in the liver, while about 95% is excreted through the lungs, resulting in minimal accumulation in the body. The boiling point (48 °C) allows isoflurane to be used at room temperature, facilitating administration with the use of vaporizers adapted to conventional ventilators used in the ICU.12
Thanks to its efficacy and safety profile, and its approval in Spain, isoflurane is the drug recommended for inhaled sedation in the ICU.13 In contrast to sevoflurane, no limit on the number of days of administration has been established, though periodic re-evaluation is advised after 48 h, due to the existence of limited data beyond this period.
SevofluraneIf isoflurane is not available, sevoflurane is seen as an alternative.14 Its time to onset of action is two minutes. Approximately 5% of the drug is metabolized in the liver, and excretion occurs through the lungs. The prolonged administration of isoflurane has been associated with nephrogenic diabetes insipidus and hypernatremia. This adverse effect is related to the main metabolite of the drug, hexafluoroisopropanol (HFIP), with the release of inorganic fluoride and CO2.15 Thus, the use of sevoflurane in the ICU is not advised for more than 5 days.16
Indications, contraindications and adverse effectsThe inhaled agents have a favorable pharmacokinetic profile for the sedation of patients in the ICU subjected to MV, thanks to their rapid onset and end of action, low liver metabolism, scant renal elimination and limited systemic accumulation. These characteristics afford great precision in controlling sedation, with stable levels most of the time, and rapid and predictable patient recovery.17
How do the inhaled agents affect awakening time and stay in the ICU?Two meta-analyses and systematic reviews of randomized prospective studies demonstrated a significant reduction in awakening time and time to extubation with inhaled agents versus propofol and midazolam.18,19 This reduction was particularly notorious versus midazolam. There were no significant differences in ICU stay or hospital stay, however. More recently, Meiser et al.,20 in a randomized, controlled, multicenter non-inferiority clinical trial of isoflurane versus propofol during 48 h in patients subjected to MV demonstrated shorter awakening times and greater spontaneous breathing rates with the former drug, with no differences in the primary endpoint (percentage of time at the desired sedation target). Subsequently, Bracht et al.,21 in a post hoc analysis with a follow-up period of 30 days, recorded more ICU-free days in the isoflurane group, and a lesser use of other sedative drugs.
Do the inhaled agents affect other analgosedation drugs and neuromuscular blockers?A lesser consumption of analgosedation drugs and neuromuscular blockers (NMBs) has been reported with the use of inhaled agents, due to their analgesic properties and interaction with the NMBs.22 Grasselli et al. recorded a lesser use of opioids and NMBs, with no greater incidence of hemodynamic adverse effects.23 This “opioid-sparing” effect is important for minimizing the complications of these drugs, such as tolerance, ileus, withdrawal syndrome, critical illness polyneuropathy , and delirium. Close monitoring of the levels of analgesia and neuromuscular blockade is required to avoid overdosing on unnecessary drugs and thus reduce the number of adverse events.
What are the cardiac effects of the inhaled agents?The inhaled agents exert a direct cardioprotective effect by facilitating the opening of ATP-dependent calcium channels and increasing the production of nitric oxide (NO) during ischemic pre- and post-conditioning. These properties have caused led them to their ptreference in cardiac surgery over intravenous anesthetics such as propofol.24,25
What are the cerebral effects of the inhaled agents?The inhaled sedatives inhibit seizure activity by inhibiting NMDA excitotoxicity and activating GABA receptors. As a result, they have been used as rescue medication in cases of refractory and super-refractory status epilepticus (RSE/SRSE).26 The reported advantages of inhaled sedatives over other intravenous sedatives include their rapid diffusion and distribution within the central nervous system, an ultra-rapid onset of action, and a short half-life. These characteristics allow excellent monitoring and titration of the drug guided by point-of-care electroencephalography (EEG), with the absence of interactions with other drugs. The concentrations required for seizure control are greater than those needed for deep sedation, with an end-tidal isoflurane concentration of between 0.8 and 2%.27 However, the reports on seizure control and the results obtained with isoflurane in RSE/SRSE are limited and further studies are needed in this respect.
On the other hand, a neuroprotective mechanism has been described in ischemic cerebrovascular disorders similar to that observed in cardiac ischemic pre- and post-conditioning. However, the decreases in mean blood pressure and cerebral perfusion pressure imply that these drugs are contraindicated in patients with intracranial hypertension.28
What are the pulmonary effects of the inhaled agents?The inhaled sedatives have been widely investigated at pulmonary level, particularly in the context of the COVID-19 pandemic.29 In addition to their direct bronchodilatory action, they have been reported to offer pneumoprotective effects that could be of benefit to patients with acute respiratory distress syndrome (ARDS). These effects comprise a decrease in the release of inflammatory mediators and a strengthening of the alveolar membrane-capillary junctions that would contribute to preserving lung integrity, reducing ventilator-induced lung injury (VILI).30 In addition, the use of inhaled sevoflurane could improve oxygenation and reduce endothelial damage and inflammatory markers in patients with ARDS, compared with midazolam.31 These inhaled agents may also be regarded as rescue medication in situations of severe refractory status asthmaticus,32 with multiple suggested beneficial mechanisms such as relaxation of the bronchial smooth muscle and inhibition of the release of inflammatory mediators.30 In sum, inhaled sedation could reduce ventilator-induced lung injury not only through the synchronization and limitation of deleterious efforts on the part of the lungs, attributed to all intravenous sedatives but also through a direct lung protective effect.33
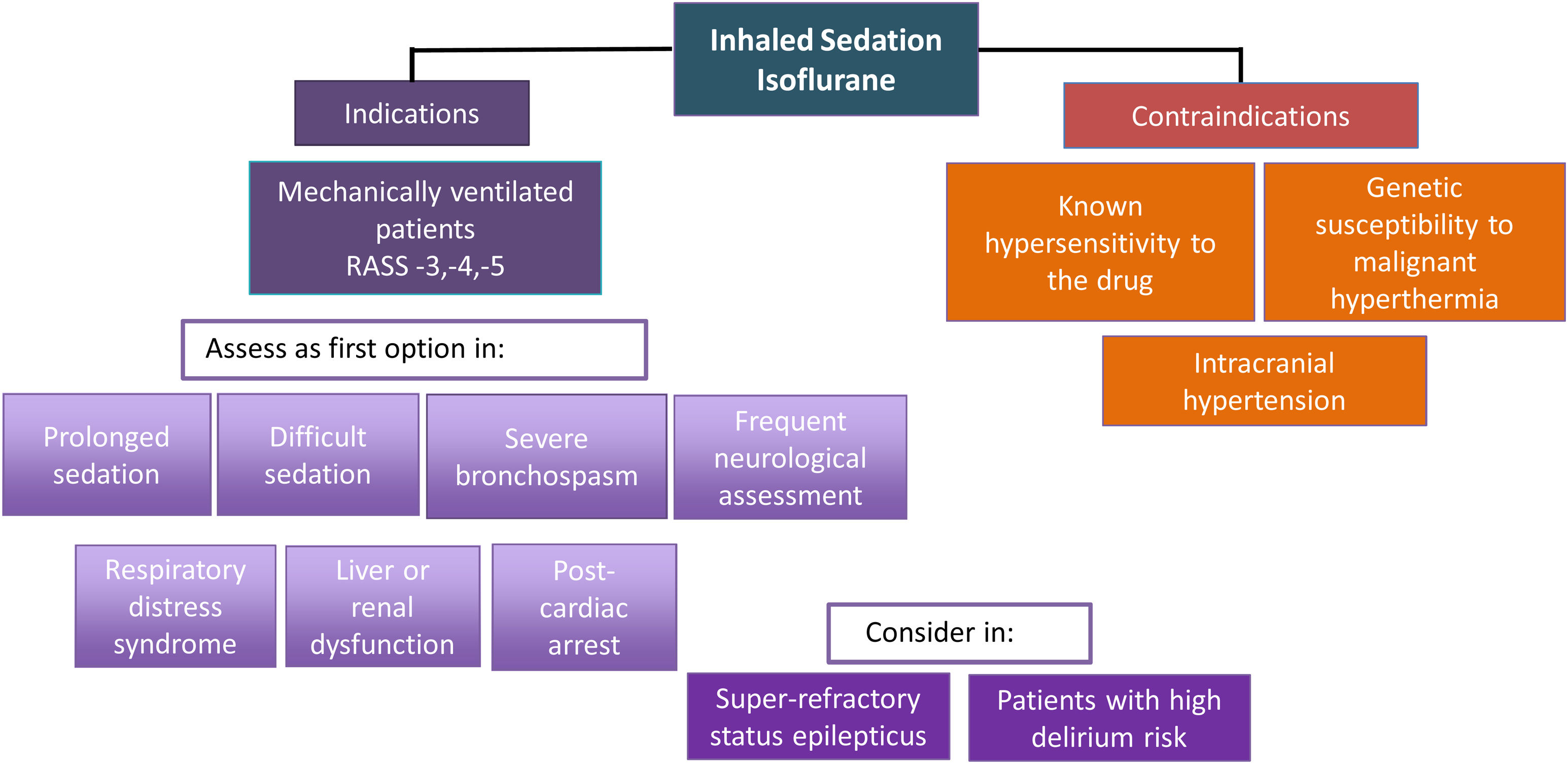
Should inhaled sedation replace intravenous sedation as the first choice in certain kinds of patients?Considering the abovementioned scientific evidence on the beneficial effects of the inhaled agents and the recommendations of the NICE guidelines,34 the GTSAD suggests that inhaled sedation with isoflurane could be regarded as first-line treatment in the following clinical scenarios, provided the necessary resources and training are available (Fig. 1):
- •
Moderate to deep sedation for any reason.
- •
Prolonged sedation to avoid side effects due to the accumulation and tachyphylaxis of other sedatives administered via the intravenous route, particularly if high doses are required.
- •
Difficult sedation, where despite maximum doses of a sedative administered via the intravenous route or the need to combine several sedatives at high doses, the target score on the Richmond Agitation-Sedation Scale (RASS) cannot be maintained within the range of −3 to −5.
- •
Severe bronchospasm complicating MV.
- •
Liver and kidney dysfunction.
- •
Patients recovered from cardiac arrest who need moderate to deep sedation.35
- •
Patients requiring frequent neurological assessment.
- •
Patients with ARDS.
- •
Patients with RSE/SRSE, as rescue therapy and/or an alternative to other sedating agents.
- •
Elderly patients at high risk of suffering delirium who require prolonged deep sedation to avoid the delirium-producing effects of other sedatives (midazolam and propofol) and opioids, through the resulting drug-sparing effect obtained.36
Isoflurane is contraindicated in patients with known hypersensitivity to volatile halogenated anesthetic agents and in patients with a known or suspected genetic susceptibility to suffer malignant hyperthermia.37 It is also contraindicated in ICH.28,38 In patients without ICH but at risk of developing it, isoflurane should only be used with neuromonitoring. Other contraindications should be considered before starting to administer the drug (Table 1. Supplementary material).
What are the associated adverse effects and how are they managed in clinical practice?Fig. 2 describes the adverse effects and potential problems that may arise in clinical practice regarding the use of isoflurane, along with the possible solutions.
Description of the techniqueHow are the halogenated agents administered in the ICU?The halogenated agents are administered through inhalation. Specific vaporizing devices are used for this purpose, designed for use in the ICU. Two systems have been described: Sedaconda-ACD® (Sedana Medical, Uppsala, Sweden)39 and the MIRUS® system (Pall Medical, Dreieich, Germany).40 The Sedaconda-ACD® device is the most widely used option and the only system to have been approved in Spain.
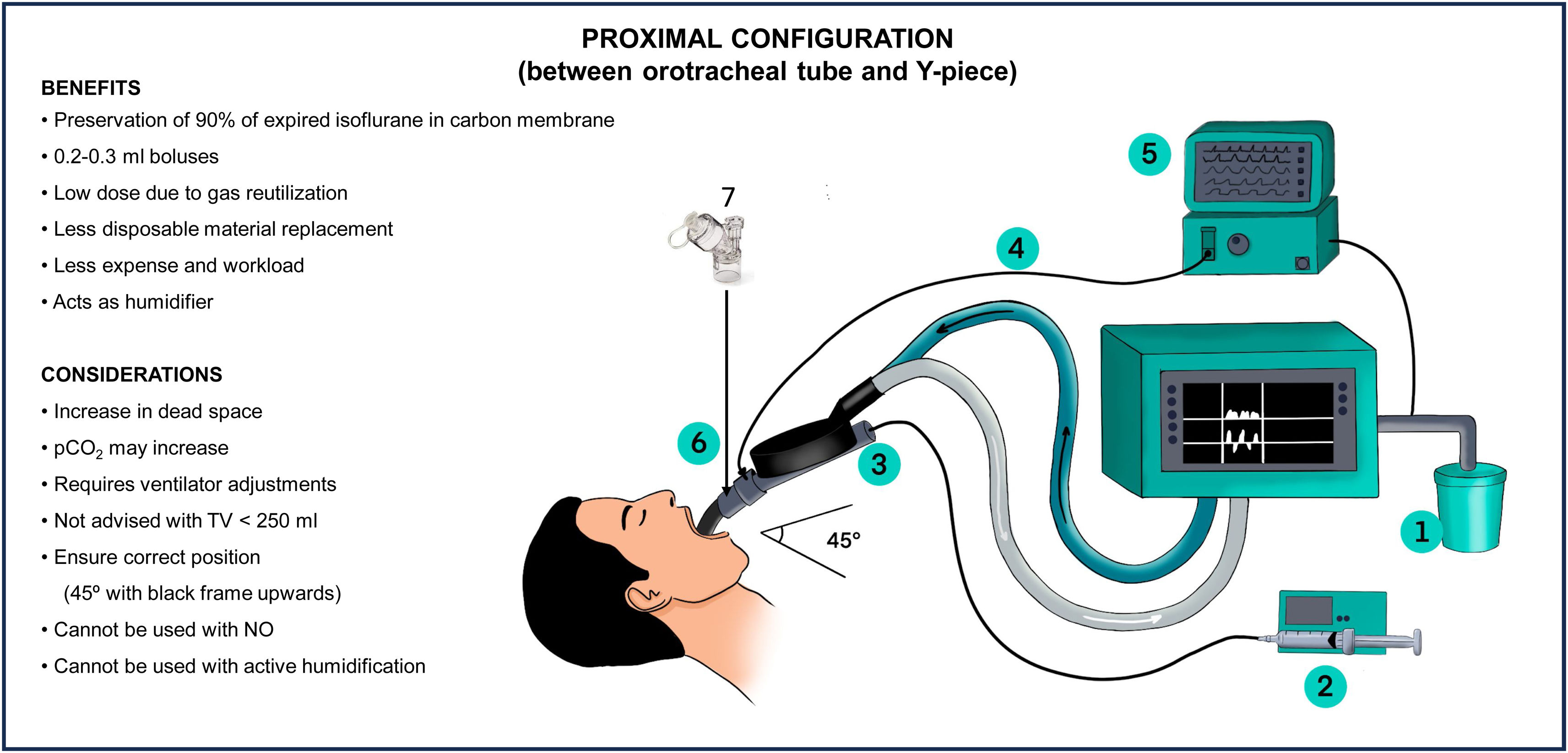
How does the Sedaconda-ACD® device work?Proximal configurationThe Sedaconda-ACD® device works as a heat and moisture exchanger (HME) with antibacterial and antiviral properties when placed between the Y-piece of the ventilator and the orotracheal tube. It provides passive humidification at a rate of 31 mg/l. The device also includes a vaporizing rod and an activated charcoal membrane that acts as a reflector.41
A passive adsorption system fitted with an activated charcoal filter is placed at the ventilator gas outlet. This filter retains the residual gas and prevents environmental contamination (Fig. 3).
During inspiration, the gas in the liquid phase administered through a syringe pump turns into vapor and mixes with the air and oxygen supplied by the ventilator. In the expiration phase, 90% of the volatile anesthetic condenses on the surface of the activated charcoal fibers and is retained as a result. Only 10% of the inhaled anesthetic agent crosses the filter and is finally eliminated to the exterior through the expiratory outlet of the ventilator42(Fig. S1 Supplementary material).
In this configuration, the device generates an increase in dead space of 50 ml.43
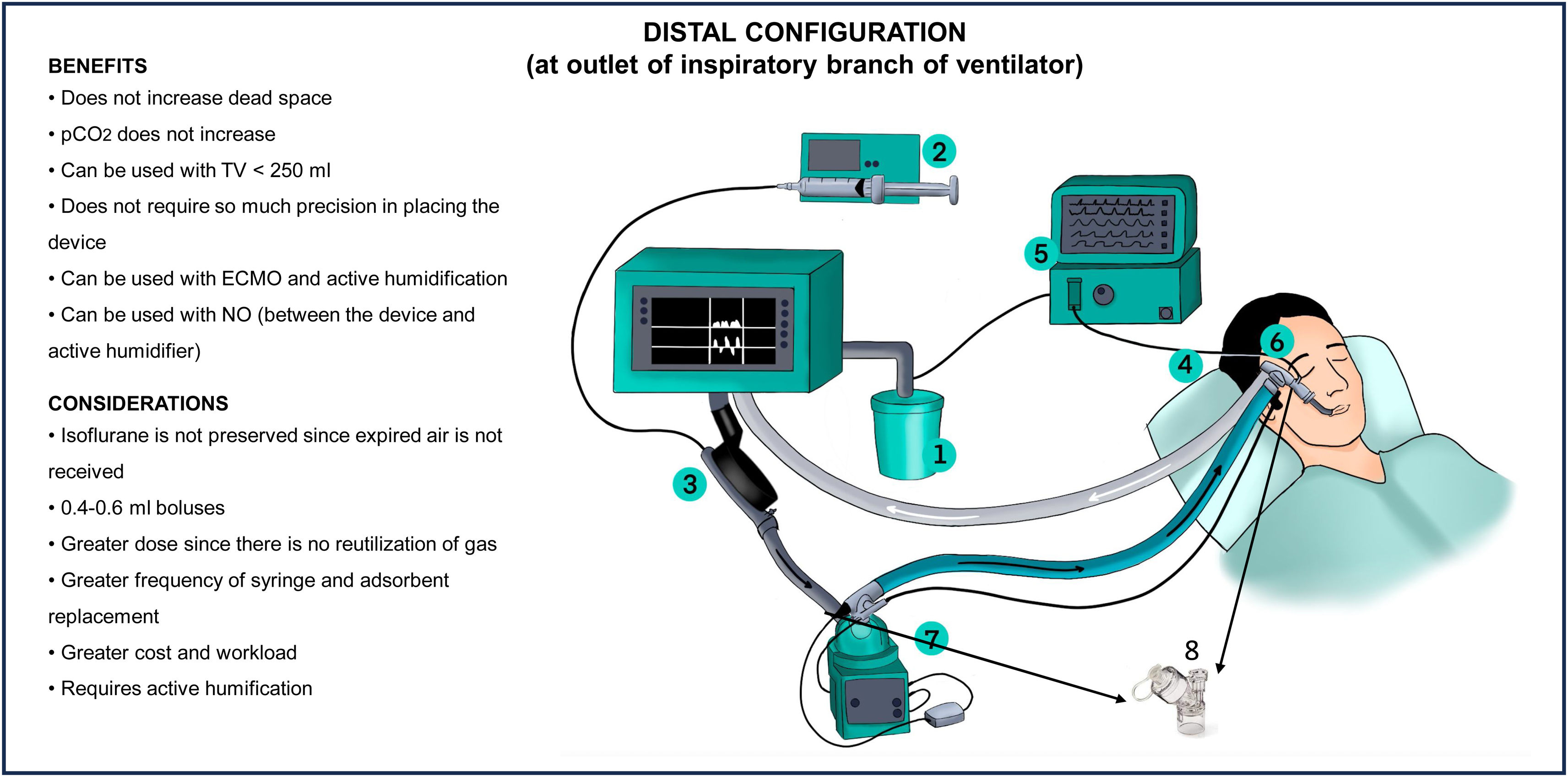
Distal configurationThe distal configuration can be used as an alternative to the proximal configuration in certain situations. Firstly, when the re-inhalation of CO2 or the 50 ml dead space of the device generates respiratory acidosis that cannot be corrected by adjusting the ventilator or replacing the device every 12 h. Secondly, this configuration is preferred when the patient is ventilated with a tidal volume of less than 250 ml. In patients subjected to ECMO, where it is not possible to adjust the gas flow to compensate the respiratory acidosis, the distal configuration should also be considered.23
In this configuration, the device is positioned at the outlet of the ventilator´s inspiratory branch, acting exclusively as a vaporizer. This configuration avoids the increase in dead space, and thus the potential increase in CO2. However, by losing the humidification capacity, active humidification must be used. This configuration also implies a significant increase in gas consumption, as t it is not re-inhaled. According to the manufacturer, this is the configuration recommended for the use of nitric oxide (NO) (Fig. 4)
How does the MIRUS™ device work?The MIRUS™ device has a control unit and specific reservoir for the anesthetic, connected to a 100 ml interface through a multi-lumen cable inserted between the Y-piece and the endotracheal tube. This interface, equipped with a reflector and filter, includes cables for the injection of gas and the measurement of pressure, flow and concentration of the gas. In addition, the filter acts as a heat and moisture exchanger, providing protection against bacteria, viruses and particles.
In order to maintain precise control, the MIRUS™ constantly regulates the pressure, flow and concentration of the gas. It samples and reintroduces exhaled gas, injecting the anesthetic vapor in pulsatile mode at the start of inspiration to ensure rapid dilution and effective transport. The end-expiratory concentration is adjusted automatically according to the target values, and the control velocity is adapted to the anesthetic agent´s clearing needs.44
Considerations during the use of inhaled sedationDuring the initiation, maintenance and withdrawal of inhaled sedation, it is essential to consider certain aspects of the devices and drugs used. In this respect, it is important to underscore the need for close monitoring to avoid the risk of oversedation.45
What monitoring is recommended during the administration of inhaled sedation?Monitoring and adjustment of the depth of sedation should follow the usual protocol, based on validated scales such as the Richmond Agitation- Sedation -Agitation Scale (RASS).46 In situations of deep sedation (RASS −4, −5), or with the use of NMBs, it is advisable to adopt monitoring with EEG, using a bispectral index (BIS) of 40–6047 or a Patient State Index (PSI) of 25–50.48
It may be useful to have an exhaled gas analyzer. Although this does not replace the evaluation of the depth of sedation, it allows us to know the concentration of exhaled gas, and is particularly useful in situations that require high concentrations to achieve bronchodilatory or antiseizure effects. In addition, it facilitates monitoring of the exhaled concentration of CO2, which helps detect the appearance of hypercapnia.49
What factors should be considered during the start and maintenance of inhaled sedation?Three main factors should be considered to optimize the dose and maintenance of inhaled gases:
- 1
The configuration of the device, whether proximal or distal.
- 2
The ventilator tidal volume.
- 3
The age of the patient.
Configuration of the device. The administered dose depends on the infusion speed of the liquid anesthetic that reaches the device to be vaporized and delivered to the patient in each inspiration.
In the proximal configuration, the starting rate of isoflurane to secure moderate to deep sedation is generally 3 ml/h (0.3−0.7%V), while in the case of sevoflurane it is 5 ml/h (0.5–1 %V).49 These rates should be adjusted every 5−10 min until the desired target is reached, and use can be made of 0.2−0.3 ml boluses.
In the distal configuration, since the gas is not reused, the infusion rates to achieve moderate to deep sedation are significantly higher: between 5−7 ml/h for isoflurane and 10−15 ml/h for sevoflurane, with boluses of 0.4−0.6 ml.
Tidal volume of the ventilator. Any modification of the tidal volume (TV) will affect the expired fraction of gas, and this consequently will require an adjustment of the infusion rate. An increase in TV will demand an increase in infusion rate, while a decrease in TV will require a decrease in rate to keep the sedation level constant.50
Age of the patient. The minimum concentrations required to reach the desired target may vary particularly with the age of the patient; in this regard, older patients have lower gas requirements.12
Special situations during maintenanceHow should transfers be made during inhaled sedation?Different strategies are available during transfers to the operating room or for complementary tests. The device can be used with the syringe if the procedure is expected to last over 20 min. In the proximal configuration, it is also possible to only use the device, without the syringe, considering that the sedative accumulated in the device has an approximate duration of 20 min, complemented with intravenous sedative boluses if necessary. This option is not feasible in the distal configuration since the effect ceases immediately upon stopping the syringe. Another option is to do without the device and administer intravenous sedatives in boluses.
How should nebulization be made during inhaled sedation?In nebulization during inhaled sedation, it is essential to consider the viscosity of the drugs and the frequency of administration to avoid obstruction of the membrane of the device. It is advisable to place the nebulizer between the orotracheal tube and the device, prioritizing on synchronized nebulization. In the distal configuration, it can also be placed between the inspiratory dry arm and the active humidifier (Figs. 3 and 4). Ultrasound and mesh humidifiers do not affect the concentration of the inhaled sedative.
Occupational exposure and safetyIs the administration of inhaled sedation in the ICU safe?Several studies have confirmed the safety and absence of significant exposure for healthcare professionals during inhaled sedation.18,51–53 Evaluation of the environment is made through air concentrations reported as parts per million (ppm), regulated in Spain by the Ministry of Labor and Social Affairs. The established daily environmental exposure limit is 50 ppm in the course of an 8 -h work shift.
What measures are recommended to minimize exposure and guarantee the safety of healthcare professionals?The recommendations that contribute to maintaining a safe environment for healthcare professionals include the following54:
- •
Adequate healthcare professional training to guarantee correct manipulation of the material and connections.
- •
Gas extraction systems with activated charcoal filters connected to the ventilator expiratory outlet, which are replaced on a protocolized basis.
- •
Minimization of procedures that can generate concentration (ppm) peaks.
- •
Closed aspiration systems in patients requiring frequent aspiration.
- •
ICU cubicles with adequate ventilation systems ensuring a minimum of 8 air renewals per hour.
Inhaled sedatives can be used effectively and safely in the ICU. Inhaled sedation offers certain advantages concerning intravenous sedation, fundamentally in the form of shorter awakening times that can facilitate frequent neurological evaluations and awakening and spontaneous breathing tests.
Based on the current scientific evidence, isoflurane may be regarded as a first-line sedative for ventilated patients who require moderate or deep sedation.
Adequate training of ICU professionals is essential for optimizing the administration of inhaled sedation and minimizing the associated risks. Therefore, continuous research and training in this field are essential to improve clinical practice and the care of critically ill patients.
Authors’ contributionSofía Contreras: development and correction of the manuscript, and approval of the final version.
Carola Giménez-Esparza Vich: development and correction of the manuscript, and approval of the final version.
Jesús Caballero: development and correction of the manuscript, and approval of the final version.
FundingNone.
AcknowledgementsNone.
Conflict of interestThe authors have collaborated in educational activities with Sedana Medical.
The following is Supplementary data to this article: