Transcranial Doppler ultrasound is able to demonstrate cerebral circulatory arrest associated to brain death, being especially useful in sedated patients, or in those in which complete neurological exploration is not possible.
Transcranial Doppler ultrasound is a portable, noninvasive and high-availability technique. Among its limitations, mention must be made of the absence of acoustic windows and false-negative cases. In patients clinically diagnosed with brain death, with open skulls or with anoxia as the cause of death, cerebral blood flow can be observed by ultrasound, since cerebral circulatory arrest is not always synchronized to the clinical diagnosis. The diagnostic rate is therefore time-dependent, and this fact must be recognized in order to avoid delays in death certification.
Despite its limitations, transcranial Doppler ultrasound helps solve common diagnostic problems, avoids the unnecessary consumption of resources, and can optimize organ harvesting for transplantation.
El Doppler transcraneal permite demostrar la parada circulatoria cerebral que acompaña a la muerte encefálica, siendo especialmente útil en pacientes sedados, o en los que no puede realizarse la exploración neurológica completa.
El Doppler transcraneal es una técnica portátil, no invasiva y de alta disponibilidad. Entre sus limitaciones está la ausencia de ventana sónica y los casos falsos negativos. En pacientes con diagnóstico clínico de muerte encefálica, que tienen cráneos abiertos o anoxia como causa de la muerte, puede sonorizarse flujo sanguíneo cerebral, ya que la parada circulatoria cerebral no siempre es sincrónica con el diagnóstico clínico. Su rentabilidad diagnóstica es, por tanto, dependiente del tiempo, hecho que debe ser reconocido para no retrasar la declaración de muerte.
A pesar de sus limitaciones, el Doppler transcraneal ayuda a resolver frecuentes problemas diagnósticos, evita un consumo innecesario de recursos y puede optimizar la obtención de órganos para trasplante.
Brain death (BD) is defined as irreversible cessation of the brain and brainstem functions. Its diagnosis has great medical, ethical and legal implications, since it represents certification of the death of the patient and therefore allows withdrawal of all the artificial support measures, or organ harvesting for transplantation purposes. Cerebral circulatory arrest (CCA) that accompanies BD occurs when the intracranial pressure (ICP) exceeds the systolic blood pressure of the patient. The diagnosis of BD is based on an exhaustive neurological exploration that must abide with certain international standards.1,2 Instrumental tests may be obligate in some clinical cases, and vary according to the legal specifications in force in each individual country.3,4 If the patient is sedated, suffers serious craniofacial damage, or presents intolerance of the apnea test, techniques that study brain circulatory flow, such as angiography with multislice computed tomography (CT), brain scintigraphy with Tc99-HMPAO, or transcranial Doppler (TCD) are advised.5–11 The diagnosis of BD is a key element in all national transplant programs. In Spain, almost 90% of all transplants are performed with organs obtained from donors that have died under conditions of BD.
Transcranial Doppler ultrasound is one of the most widely used methods for diagnosing BD, and is legally accepted in Spain.12 Knowing its advantages and limitations can help resolve frequent diagnostic problems in the intensive care unit (ICU), avoid unnecessary resource consumption, and optimize the obtainment of organs for transplantation.
Transcranial Doppler and the legal diagnosis of brain deathSpanish Royal Decree (RD) 1723/2012, under Annex 1, Protocols referred to the diagnosis and certification of death for the obtainment of dead donor organs,12 specifies the clinical circumstances in which instrumental tests are obligate. In its Section 4, and among the “tests evaluating cerebral blood flow”, TCD is described as one of the techniques that can be used, though the Decree does not specify which cerebral arteries are to be explored, or what type of ultrasound findings are needed to confirm CCA. As specified in Section 3 of the mentioned Decree, TCD allows abbreviation or even suppression (according to medical criterion) of the recommended 6-h observation period in destructive brain damage and 24h of anoxia, thereby facilitating things in the event of organ donation.
Transcranial Doppler in cerebral circulatory arrestPatients with serious brain damage and refractory intracranial hypertension syndrome suffer a gradual decrease in brain perfusion pressure and cerebral blood flow, which finally causes CCA. The use of TCD for the diagnosis of CCA was first described in 1974,13 and since then many publications have warranted its usefulness in this context.1,14,15
The study of cerebral blood flow velocity with TCD during the development of CCA distinguishes four evolutive stages,5 each with its own characteristic flow pattern:
- 1
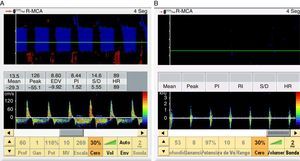
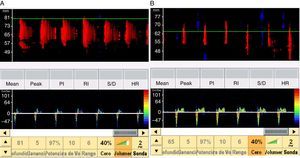
When the ICP exceeds the diastolic blood pressure, the cerebral blood flow velocity at the end of diastole is zero, and flow persists only during systole. The mean velocity is >10cm/s, and there is still some net flow, with a very high pulsatility index. These findings are recorded in situations of severe intracranial hypertension and may be taken to represent a pre-CCA pattern (Fig. 1).
- 2
When the ICP is equal to or greater than the systolic blood pressure of the patient, brain perfusion ceases. This phase is characterized by the appearance of a pattern known as reverberant flow, biphasic oscillating flow or inverted diastolic flow (Figs. 2 and 3), produced by the elasticity of the arterial wall. It involves anterograde flow in systole, and retrograde or inverted flow in diastole–both being approximately equal in the same cardiac cycle, and the net brain flow is zero. This pattern has a brief systolic phase. All these observations are correlated to CCA in the arteriographic study.16
- 3
When the ICP exceeds the systolic blood pressure of the patient we only record systolic spikes or systolic spicules, which are small, anterograde sharp systolic waves with a duration of <200ms and with a systolic peak velocity of <50cm/s. Flow is likewise lacking during the rest of systole and in diastole of the cardiac cycle (Fig. 2).
- 4
In very advanced cases, with great ICP elevations, we observe flow obstruction in the more proximal segments of the arteries of the skull base, causing a total absence of flow signals. In these cases the doubt may arise as to whether the absence of signals is due to CCA or to the lack of an acoustic window. In order to accept the absence of a signal as criterion of CCA, the TCD exploration must be performed under the same clinical conditions and by the same expert explorer as in the previous studies in which flow was still observed.
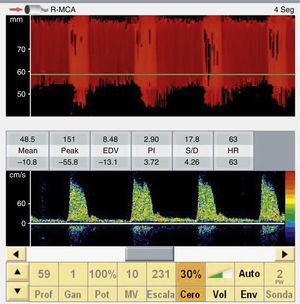
The presence of effective flow in any intracranial artery completely discards CCA (Fig. 3).
Doppler study in the extracranial arteriesOne of the main limitations of TCD is the absence of an acoustic window, which prevents us from obtaining intracranial signals–a situation that is found in about 10–20% of the population17,18 and which is more common in elderly females. In these cases we can study the internal carotid artery (ICA) and the vertebral arteries (VAs) at extracranial level. When access through the temporal window is not possible, some authors use the ophthalmic window to study the internal carotid artery at carotid siphon level.19,20 In this way, Lampl et al.19 were able to improve the diagnostic performance of the technique by 9%. The use of echo-enhancers also improves exploration in cases of a poor acoustic window. The presence of flow in the carotid siphon is explained by the shunting of blood from the external carotid artery or by CCA occurring distal to it.
The presence of flow in the internal carotid artery and extracranial vertebral arteries does not discard CCA. Consequently, only the transcranial study and the presence of systolic spikes or inverted diastolic flow in the arteries of the skull base are of diagnostic usefulness in relation to BD.
Conditions for establishing a diagnosis of cerebral circulatory arrest using transcranial Doppler ultrasoundAn adequate system is required, equipped with a 2MHz pulsed-Doppler probe and involving a study sample volume of under 10mm, with the capacity to reach a transmission power of 100mW/cm2. The explorer must be an expert, and some guides recommend performing two explorations spaced 30min apart in order to guarantee the irreversibility of CCA.6,8
The consensus conference on the diagnosis of CCA, held by a task force of the Neurosonology group dependent upon the World Federation of Neurology,5 together with the recommendations of the Spanish Society of Neurology,7 require the patient to be hemodynamically stable with a mean blood pressure (MBP)≥70mmHg (blood pressure no lower than 90/50mmHg), and PaCO2 35–45mmHg. Other prerequisites are knowledge of the cause of coma, and the exclusion of hypothermia, metabolic alterations, intoxications and other factors that may alter the neurological findings. Furthermore, two expert physicians are required to determine the absence of brain functions. These conditions seem very restrictive, since one of the main advantages of TCD is its capacity to diagnose CCA in sedated patients or in cases posing clinical diagnostic problems. What advantage could TCD offer if the patient diagnosis has already been confirmed? In our opinion, the requirements of the task force5 and the recommendations of the Spanish Society of Neurology7 regarding a complete clinical diagnosis before accepting Doppler ultrasound as a criterion for diagnosing CCA represent unjustified diagnostic demands that deserve to be reconsidered.
In diagnosing CCA, use is made of both TCD, which is a blind technique, and transcranial color-coded duplex sonography,21–23 which offers more advantages, but which is less widely used in ICUs. Transcranial color-coded duplex sonography allows simultaneous two-dimensional study of the brain (with visualization in color of the arteries and veins of the skull base) and analysis of the vascular Doppler spectra. The transcranial color-coded angioduplex mode, together with the use of echo-enhancers, allows greater percentage vascular identification than conventional TCD. Because of its advantages, it probably will become the most widely used technique in the future.
Transcranial Doppler ultrasound and the diagnosis of brain deathIn order to diagnose BD using TCD, we must confirm CCA based on the bilateral recording of reverberant or inverted diastolic flow and systolic spikes in the anterior and posterior circulation.1,14 These findings must be demonstrated by exploring through the temporal window (both middle cerebral arteries; anterior circulation) and through the suboccipital window (vertebral arteries and basilar artery; posterior circulation)–though some authors consider exploration of the basilar artery to be sufficient.23 The advantages and limitations of TCD in diagnosing CCA associated to BD are described in Table 1.
Advantages and limitations of transcranial Doppler in diagnosing brain death.
| Advantages |
| Portable technique allowing patient bedside explorations |
| Noninvasive method measuring intracranial arterial flow velocity |
| Allows monitoring of patient evolution |
| Relatively inexpensive |
| Widely available |
| Very specific |
| Results not interfered with by central nervous system depressor drugs |
| Limitations |
| Explorer dependent technique |
| Absence of acoustic window, which prevents ultrasound transmission (10–20%) |
| Difficulty exploring posterior circulation in critical patients |
| False-negative results in patients with anoxia |
| False-negative results in patients with open skull (decompressive craniectomy, fractures with skull dome rupture, ventricular drainage, and infants with open fontanelles) |
| Time-dependent diagnostic performance (diagnostic sensitivity increases over time) |
A metaanalysis published in 20066 evaluated a total of 684 patients included in 10 studies covering the period 1980–2004. Only two of the studies were considered to be of high quality, and these two articles reported a sensitivity of 95% and a specificity of 99%. On including the other 8 studies of lesser quality in the analysis, the sensitivity was seen to decrease to 88%, while specificity remained the same (99%). Of note in this review is the many studies that did not explore the posterior fossa. This must be criticized, since a diagnosis of BD requires the confirmation of CCA by exploring both the anterior and the posterior circulation. Ultrasound exploration of the posterior circulation in an intubated patient involves some technical difficulty, and therefore the high sensitivity obtained in the mentioned metaanalysis can be explained by the many studies that failed to explore the posterior fossa. The specificity of TCD as a test for confirming BD in the literature varies between 97 and 100%.6,11,14
Limitations of transcranial Doppler in diagnosing brain death- -
Absence of an acoustic window.
- -
Explorer dependency.
- -
Difficulty exploring the posterior circulation.
- -
False-positive readings in diagnosing BD (patients who do not meet the clinical criteria of BD but present CCA at Doppler exploration): ultrasound patterns consistent with CCA have been described in the early stage of subarachnoid hemorrhage secondary to aneurysmal rupture with large ICP elevations, and in cardiac arrest. These situations are transient and reversible; some guides therefore recommend repeating the exploration after 30min.5,7 However, this general norm does not appear to be very justified in patients with refractory and progressive intracranial hypertension syndrome, in which daily TCD monitoring is performed, and gradual worsening until reaching criteria of CCA is confirmed. In addition to these transient situations, there have been some exceptional reports of false-positive readings in both open-skull patients and in patients without cranial defects. In all of these cases TCD exploration was complete, comprising both the anterior and the posterior circulation.8,24,25
- -
False-negative readings in diagnosing BD (patients with clinical criteria of BD and the presence of flow at Doppler exploration): open-skull patients may have clinical criteria of BD in the presence of cerebral blood flow. This situation can be seen in patients with a ventricular drain, decompressive craniectomy, skull dome rupture, fractures of the base of the skull, and in infants under 1 year of age with still open fontanelles. In all of these cases the open skull condition allows a certain intracranial decompression that explains the persistence of flow in some of the intracranial arteries, despite clinically confirmed BD.26–28 It is therefore very important to remember that CCA is not always in synchrony with the clinical exploration of BD, i.e., CCA is accompanied by clinical data of BD, though the opposite is not always true, since some patients present cerebral blood flow despite a clinically confirmed diagnosis of BD (these being cases of “flow without function”). This same phenomenon has been described in patients with post-cardiac arrest anoxic encephalopathy.28–30 In such cases, during the interval of cardiac arrest, the neurons suffer irreversible damage, though the recovery of heart beat with cardiopulmonary resuscitation maneuvering produces cerebral reperfusion that explains the ultrasound confirmation of flow despite the existence of neuron death. This mechanism explains the “flow without function” phenomenon in anoxia.
In these cases, the use of TCD not only fails to help in diagnosing BD but also may even complicate things by causing a delay in confirming CCA. For this reason the Spanish Neurosonology Society recommends that TCD should not be used as a complementary technique for diagnosing BD in open-skull patients.7 However, and since this persistent flow phenomenon does not always occur, we consider that the use of TCD can be defended, bearing in mind these limitations, with a view to avoiding delays in the diagnosis of BD and the certification of patient death. If there are no problems or interferences in establishing a clinical diagnosis of BD, then the clinical exploration should prevail over the TCD findings. The presence of “flow without function” in open-skull patients and a clinical diagnosis of BD has also been demonstrated in other flow studies such as cerebral angiography with multislice computed tomography.30–32
The diagnosis of CCA is time-dependent: some authors have found that CCA documented by TCD (even in closed-skull patients) is not simultaneous to the clinical diagnosis of BD; its diagnostic performance is therefore regarded as being time-dependent. Dosemeci et al.33 reported a sensitivity of 70.5% in a study of 61 patients with a clinical diagnosis of BD. In the first TCD exploration performed 0.5–4h after confirmation of the clinical diagnosis, the authors recorded persistent flow in 18 patients (29%). Subsequent follow-up of these patients confirmed CCA in 12 cases at second TCD exploration 12.6±8.3h after the clinical diagnosis. Two patients required a third exploration, and another required four TCD explorations to confirm BD 96h after the clinical diagnosis. Based on these results, Dosemeci et al. concluded that the need to demonstrate CCA in patients with a clinical diagnosis of BD should be debated, since it can delay the certification of patient death. Kuo et al.34 reported a sensitivity of 77.2% in 44 patients diagnosed with BD, and likewise found the diagnostic sensitivity to increase over time. Specifically, up until 6h after the clinical diagnosis, TCD only confirmed CCA in 58.3% of the patients, while after 6–12h, 12–24h and 24–36h the percentage was seen to increase to 76.9, 83.3 and 100%, respectively.
ConclusionsExploration with TCD is noninvasive, repeatable and readily available, and allows the demonstration of CCA accompanying BD. Thanks to its specificity of close to 100%, the technique is particularly useful in sedated patients or in individuals in which a complete neurological exploration proves difficult. Its limitations comprise the absence of an acoustic window, explorer dependency, and the existence of false-negative readings (patients with blood flow at TCD exploration but with a clinical diagnosis of BD) in the presence of an open skull and anoxia. On the other hand, CCA is not always synchronic to the clinical diagnosis of BD; as a result, its diagnostic performance is time-dependent–a fact that must be taken into account in order to avoid delays in certifying patient death. Despite its limitations, TCD is an excellent tool for diagnosing BD and can optimize the obtainment of organs for transplantation purposes. An update on the diagnostic criteria of CCA, based on a multidisciplinary consensus conference, would be desirable.
Financial supportThe authors have received no financial support of any kind in relation to this study.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Escudero D, Otero J, Quindós B, Viña L. Doppler transcraneal en el diagnóstico de la muerte encefálica. ¿Es útil o retrasa el diagnóstico? Med Intensiva. 2015;39:244–250.