The administration of parenteral nutrition to critically ill patients is indicated in cases of intestinal failure that prevents the administration of enteral nutrition (EN) or in situations that condition poor supply1,2. Despite the fact that few studies have been conducted in critically ill patients, it is assumed that bowel rest associated with the exclusive use of total parenteral nutrition (TPN) damages the structure and functions of the intestinal mucosa3–5. In this context, tolerance at the beginning of enteral support could be influenced by these anatomical and functional changes. The clinical implications of these alterations have not been studied thoroughly and are barely mentioned in the clinical practice guidelines and the medical literature available. At this point, the incidence rate of complications during transition from total parenteral nutrition to enteral nutrition (TPN-to-EN) or whether it is possible to impact—through clinical practice—the tolerance of rebooting EN remains unknown.
The objective of our study is to know the routine clinical practice in the management of TPN-to-EN in critically ill patients at the intensive care unit (ICU) setting. Therefore, an anonymized survey was submitted—one time only—to the Clinical Nutrition Working Group of the Spanish Society of Hospital Pharmacy (SEFH) and the SEFH list. Also, to the Metabolism and Nutrition Working Group (GTMyN) of the Spanish Society of Intensive and Critical Care Medicine and Coronary Units (SEMICYUC) and to those Spanish hospitals interested in participating. Survey was conducted between January 20, 2021 and February 20, 2021 through an online form. Different health professionals from the same unit participated. Survey consisted of 20 questions grouped into 3 categories (Appendix B; Table 1 of the Supplementary data [SD]): participant data, definition, and degree of protocolization, and type of enteral formulation that should be used. Descriptive statistical analyses of the sample were performed. Variables are expressed as percentages.
A total of 105 health professionals in Spain responded to the survey. A total of 61.90% of respondents worked at the intensive medicine unit setting, 34.29% at the hospital pharmacy, and 3.81% at the diet or endocrinology unit. The characteristics of participants are shown in Appendix B; Table 2 of the SD.
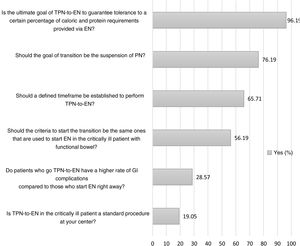
A total of 96.19% of respondents claimed that the goal of transition was to guarantee the tolerance of a certain percentage of caloric and protein requirements provided via EN (Fig. 1). Although the range of responses on which the threshold to suspend parenteral nutrition was between 40% and 100% of the theoretical enteral requirements, 74.26% of respondents considered the range to be somewhere between 60% and 75% (Appendix B; Fig. 1 of the SD). A total of 65.71% considered that a defined temporal margin should have been established to do the transition being 72h the most suggested timeframe of all (Fig. 1, and Appendix B; Fig. 2 of the SD). A total of 56.19% responded that the criteria to start EN should be the same ones that are used for critically ill patients with functional bowel (Fig. 1).
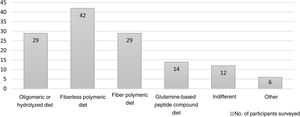
A great variability was seen in the selection of the type of diet (Fig. 2). Respondents considered that the diets to start TPN-to-EN in the critically ill patient should be fiberless polymeric diets (n=42), fiber polymeric diets (n=29), oligomeric or hydrolyzed diets (n=29), and glutamine-based peptide compound diets (n=14). In the routine clinical practice, 94.29% of respondents start EN at progressive volumes compared to 5.71% who start full doses. A total of 45.71% responded that there is scientific evidence to say that gradually increasing the volume and complexity of the diet could improve enteral tolerance (Appendix B; Fig. 3 of the SD).
During the process of transition, 61.90% of respondents said that they subtract the calories administered via enteral nutrition from parenteral nutrition by estimating and adjusting both supplies unlike 35.24% of respondents who do so on a rough basis (Appendix B; Fig. 4 of the SD). A total of 71.43% responded that, based on their own experience, patients who do this transition should not have a higher rate of GI complications compared to those who start EN early on (Fig. 1). Regarding GI complications6, they considered that the most common of all are increased gastric residual volume (n=69), abdominal distension (n=60), diarrhea (n=48), food regurgitation (n=27), vomiting (n=16), and constipation (n=15).
Our results show a significant variability among the different hospitals that participated in the survey regarding TPN-to-EN. Currently, few recommendations exist in the clinical practice guidelines and scientific literature on what the best time to start this transition should be, how to move forward with the procedure, the effect enteral diet has on tolerance or management, and the necessary duration of such procedure.
Respondents to the survey thought that transition should achieve tolerance of 60%–75% of caloric and protein requirements via enteral nutrition in a 72-h timeframe. Also, that parenteral nutrition should be removed without need for consensus on the type of enteral diet that should be used.
Process should be gradual throughout 48–72h although with variable duration and based on the characteristics of the patient and the degree of tolerance towards EN. We should mention that there was no urgent need to suspend parenteral nutrition immediately. However, the caloric and protein intakes of both nutritional supports should be closely monitored to avoid overnutrition7. Transition should be considered complete once the patient can tolerate >60%–75% of the enteral diet prescribed for, at least, 48–72h.
The development and implementation of protocols based on scientific evidence on the management of nutritional medical treatment improve clinical practice and impact the prognosis of critically ill patients8. The lack of evidence and consensus on the management of this clinical situation together with the results of this survey back up the importance of reviewing the best strategy to optimize TPN-to-EN in the critically ill patient could be.
FundingNone whatsoever.
Conflicts of interestNone reported.
We wish to thank all members from the Clinical Nutrition Working Group of the Spanish Society of Hospital Pharmacy (SEFH), and the Metabolism and Nutrition Working Group (GTMyN) of the Spanish Society of Intensive and Critical Care Medicine and Coronary Units (SEMICYUC). Also, we wish to thank those who collaborated in the spreading and design of this survey. Also, thanks to all those who participated in the survey. This study falls within the framework of a doctoral thesis conducted at Universidad Autónoma de Barcelona Medical School, Barcelona, Catalonia, Spain.
Please cite this article as: Pérez-Cordón L, Yébenes JC, Martínez de Lagrán I, Campins L. Encuesta sobre la transición de nutrición parenteral total a nutrición enteral en pacientes críticos en los hospitales de España. Med Intensiva. 2022;46:475–477.