Traumatic cerebrovascular injury (TCVI) is a rare complication of patients with traumatic disease with an incidence rate between 0.5% and 3.3%. Neurologic deficits—that can occur after the acute phase—happen in up to 58% of the patients with a mortality rate close to 25%.1,2 It is important to identify risk groups so that optimal treatment can improve functional results and reduce the morbidity and mortality rates. We describe a series of cases approved by the local research ethics committee. The patients and/or their representatives’ written informed consents were granted.
The identification of patients who can develop TCVI is one of the main challenges. Guidelines recommend using the modified Denver and Memphis criteria as screening.3,4 High-energy mechanisms are the leading cause of TCVI, above all, those causing flexion-extension, rotation, and deceleration; anecdotically, low-energy trivial mechanisms like chiropractic, and the practice of yoga have been described.5 In our series, all cases presented with closed trauma, being high-energy most of them, due to traffic accidents (60%). All showed traumatic brain injury. Clinical characteristics and severity scores are shown on Table 1.
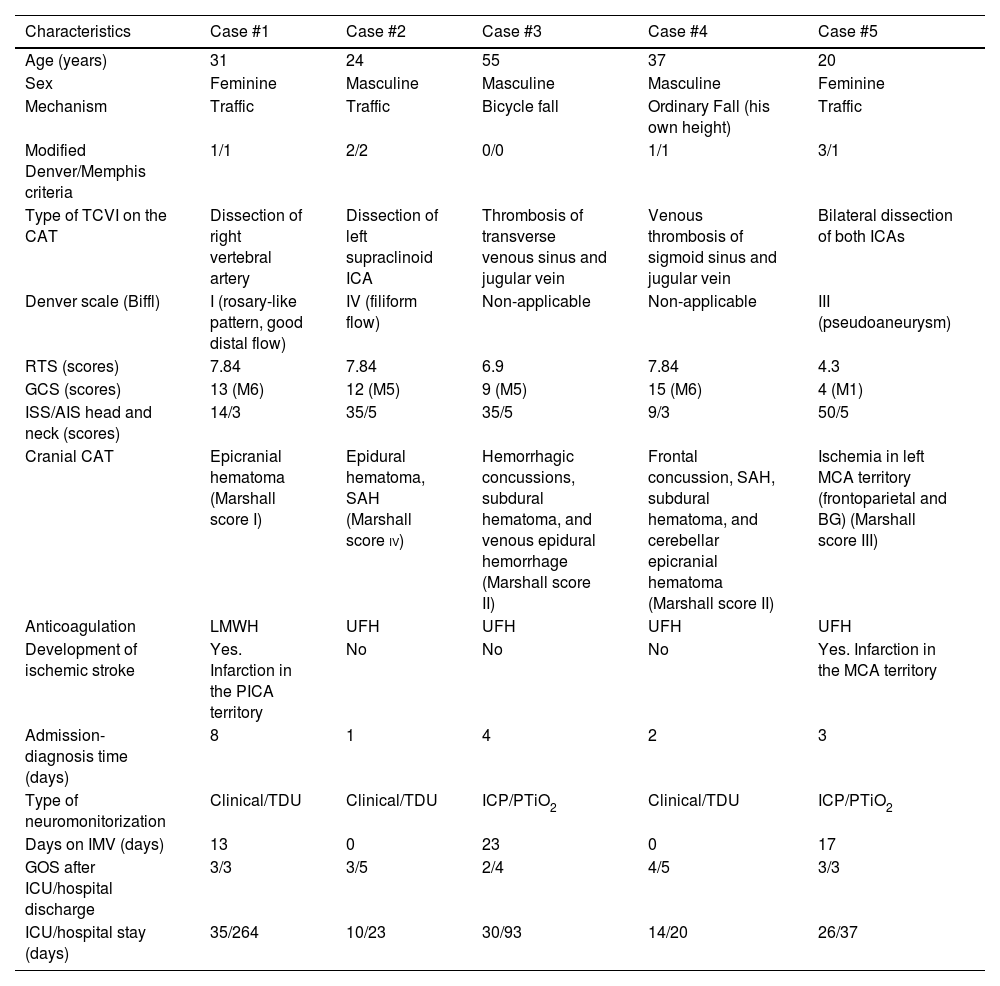
Demographic, clinical, and evolutionary characteristics of the cases.
| Characteristics | Case #1 | Case #2 | Case #3 | Case #4 | Case #5 |
|---|---|---|---|---|---|
| Age (years) | 31 | 24 | 55 | 37 | 20 |
| Sex | Feminine | Masculine | Masculine | Masculine | Feminine |
| Mechanism | Traffic | Traffic | Bicycle fall | Ordinary Fall (his own height) | Traffic |
| Modified Denver/Memphis criteria | 1/1 | 2/2 | 0/0 | 1/1 | 3/1 |
| Type of TCVI on the CAT | Dissection of right vertebral artery | Dissection of left supraclinoid ICA | Thrombosis of transverse venous sinus and jugular vein | Venous thrombosis of sigmoid sinus and jugular vein | Bilateral dissection of both ICAs |
| Denver scale (Biffl) | I (rosary-like pattern, good distal flow) | IV (filiform flow) | Non-applicable | Non-applicable | III (pseudoaneurysm) |
| RTS (scores) | 7.84 | 7.84 | 6.9 | 7.84 | 4.3 |
| GCS (scores) | 13 (M6) | 12 (M5) | 9 (M5) | 15 (M6) | 4 (M1) |
| ISS/AIS head and neck (scores) | 14/3 | 35/5 | 35/5 | 9/3 | 50/5 |
| Cranial CAT | Epicranial hematoma (Marshall score I) | Epidural hematoma, SAH (Marshall score iv) | Hemorrhagic concussions, subdural hematoma, and venous epidural hemorrhage (Marshall score II) | Frontal concussion, SAH, subdural hematoma, and cerebellar epicranial hematoma (Marshall score II) | Ischemia in left MCA territory (frontoparietal and BG) (Marshall score III) |
| Anticoagulation | LMWH | UFH | UFH | UFH | UFH |
| Development of ischemic stroke | Yes. Infarction in the PICA territory | No | No | No | Yes. Infarction in the MCA territory |
| Admission-diagnosis time (days) | 8 | 1 | 4 | 2 | 3 |
| Type of neuromonitorization | Clinical/TDU | Clinical/TDU | ICP/PTiO2 | Clinical/TDU | ICP/PTiO2 |
| Days on IMV (days) | 13 | 0 | 23 | 0 | 17 |
| GOS after ICU/hospital discharge | 3/3 | 3/5 | 2/4 | 4/5 | 3/3 |
| ICU/hospital stay (days) | 35/264 | 10/23 | 30/93 | 14/20 | 26/37 |
AIS, Abbreviated Injury Scale; BG, basal ganglia; CAT, computerized axial tomography scan; GCS, Glasgow Coma Scale; GOS, Glasgow Outcome Scale; ICA, internal carotid artery; ICP, intracranial pressure; ICU, intensive care unit; IMV, invasive mechanical ventilation; ISS: Injury Severity Score; LMWH, low-molecular weight heparin; M, motor; MCA, middle cerebral artery; PICA, posterior inferior cerebellar artery; PTiO2, brain tissue oxygen pressure; RTS, Revised Trauma Score; SAH, subarachnoid hemorrhage; TCVI, traumatic cerebrovascular injury; TDU, transcranial Doppler ultrasound; UFH: unfractionated heparin.
The modified Denver and Memphis criteria include aspects associated with the lesion mechanism, associated lesions, and the clinical characteristics of patients with traumatic disease. However, despite such criteria, a non-negligible number of patients won’t be diagnosed with TCVI.1 In this sense, a recent study conducted by Leichtle et al.6 estimates that up to 20% of the patients are misdiagnosed, and 25% of these have severity scores ≥ 3 according to the Denver scale; it is for this reason that authors recommend universal screening to discard TCVI in all patients with severe traumatic disease due to closed mechanisms. However, feasibility and cost-effectiveness studies are needed to back up this approach.
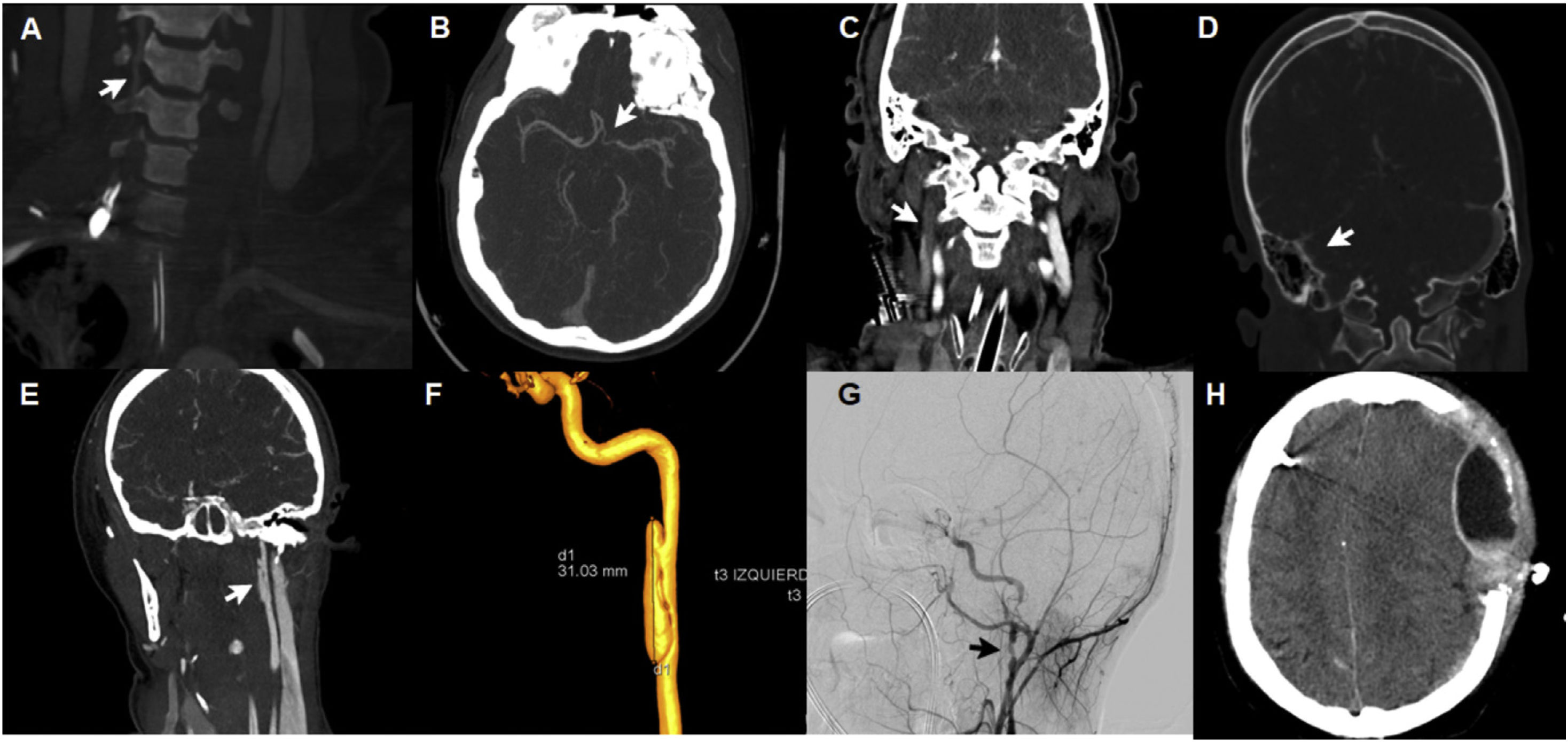
The severity of TCVI is defined based on the Denver severity scale (also called the Biffl scale), and has been designed to guide the therapeutic approach and for the prognosis of results.4 However, this scale only defines lesions caused at arterial level at the TCVI normal setting sparing venous lesions on cerebral sinuses that can be accompanied by neurologic deficits.5 We present 5 cases, 3 of which showed arterial lesions with severity grades ii, iii, and iv (Fig. 1A, B, E, F, and G) plus 2 cases of lesions in cerebral venous sinuses (Fig. 1C and D).
Radiographic aspects of patients with traumatic cerebrovascular lesion. A: dissection of right vertebral artery with rosary-like pattern and good distal flow (Denver type i). B: dissection of left supraclinoid internal carotid artery, filiform flow (Denver type iv). C: thrombosis of transverse venous sinus and right jugular vein. D: venous thrombosis of right sigmoid sinus. E: dissection of left carotid artery with development of pseudoaneurysm (Denver type iii). F: arteriography with 3D reconstruction of pseudoaneurysm (3 cm of longitudinal axis) in the left carotid artery postbulbar extracranial segment. G: dissection of postbulbar extracranial segment with presence of 2 pseudoaneurysms in the right internal carotid artery. H: hyperacute epidural hematoma after starting anticoagulation therapy.
The therapeutic goal is to prevent the development of ischemic lesions.3 Benefits in the morbidity and mortality rates have been demonstrated with the early use of antithrombotic therapy4; despite of that, there is discrepancy on what the most suitable antithrombotic therapy is (anticoagulation or antiplatelet therapy).7 The risk of ischemic events increases the severity of the lesion; therefore, the current recommendations advocate for using antithrombotic therapy after diagnosis considering the bleeding risks involved.3,4,8 In our series, 4 patients received anticoagulation with unfractionated heparin, and 1 with low-molecular weight heparin. One patient presented with bleeding (Fig. 1H) 5 days after starting anticoagulant therapy with unfractionated heparin. At the present time, no clinical trials have been conducted to guide the early use of antithrombotic therapy. Compared between the two, no therapy has been associated with a lower rate of ischemic lesions although most are low-quality studies with a high risk of bias.7 Despite all this, unfractionated heparin is often advised for its reversibility, which is essential in the acute phase in addition to modified antiplatelet therapy.3,7,9 Regarding patients with established ischemic lesions, antithrombotic therapy is not clear either. There is no evidence that dual antiplatelet therapy is more effective compared to the single-drug regime.3
Endovascular procedures are not considered a routine practice in low-grade lesions (I or II). However, they should be considered in more severe cases (grades III, IV, and V).3,4 Low-grade lesions (i, and ii) often have favorable progression; grade i lesions heal in 75% of the cases while grade ii lesions will only do so in 8% of the cases, and 30% will progress into grade i lesions. Grade i lesions progress in 8% while grade ii lesions do so in 40% of the cases.5 High-grade lesions (iii, iv, and v) often have worse progression; grade iii lesions heal or improve 11% of the times but get worse in 25% of the cases. Grade iv lesions recanalize in 40% of the cases, but most of them show no changes at all.5,10 In any case, a more severe lesion, complete occlusion or pseudoaneurysm should anticipate the use of the endovascular or surgical approach.
Overall, radiographic follow-up 7–10 days after diagnosis is advised; in case of vascular lesion resolution, the antithrombotic therapy could be suspended.3 On the other hand, with persistent lesions, keeping antithrombotic therapy and performing a new imaging modality study at 6 months would be advised to reassess the need for moving on with therapy.3,4
In our series, the ICU stay was, on average, 26 days (IQR, 14–30) while the hospital stay was, on average, 37 days (IQR, 23–93). No patient died at the ICU or hospital discharge. Regarding functional results, ischemic lesion occurred in 2 patients.
TCVI is a preventable cause for stroke; its rapid detection and proper treatment are just essential to reduce the morbidity and mortality rates associated with TCVI. We believe it is of paramount importance that neurotrauma units develop protocols for the diagnosis and management of this condition.
We wish to thank the Servicio de Medicina Intensiva del Complejo Hospitalario de Toledo, Spain.?