The Spanish Society of Intensive and Critical Care Medicine and Coronary Units (SEMICYUC) Bioethics Working Group has developed recommendations on the Limitation of Advanced Life Support Treatment (LLST) decisions, with the aim of reducing variability in clinical practice and of improving end of life care in critically ill patients.
The conceptual framework of LLST and futility are explained. Recommendations referred to new forms of LLST encompassing also the adequacy of other treatments and diagnostic methods are developed. In addition, planning of the possible clinical courses following the decision of LLST is commented. The importance of advanced care planning in decision-making is emphasized, and intensive care oriented towards organ donation at end of life in the critically ill patient is described. The integration of palliative care in the critical patient treatment is promoted in end of life stages in the Intensive Care Unit.
El Grupo de Trabajo de Bioética de la SEMICYUC ha elaborado las recomendaciones en la toma de decisiones de limitación de tratamientos de soporte vital (LTSV) con la aspiración de disminuir la variabilidad en la práctica clínica observada y de contribuir a la mejora de los cuidados al final de la vida del paciente crítico. Además de abordar el marco conceptual de la LTSV y de la futilidad, desarrolla las nuevas formas de limitación extendiéndola a la adecuación de otros tratamientos y métodos diagnósticos, además de planificar los posibles cursos evolutivos tras la decisión de LTSV. Se enfatiza la importancia de la planificación compartida de la asistencia sanitaria en la toma de decisiones, se presentan los cuidados intensivos orientados a la donación y se promueve la integración de los cuidados paliativos en el tratamiento del paciente crítico en estadios del final de la vida en UCI.
A decade ago the Bioethics Working group (WG) of the Spanish Society of Critical Care Medicine and Coronary Units (SEMICYUC) published the Recommendations for End-of-life Treatment in the Critically Ill Patient (Recomendaciones de Tratamiento al Final de la Vida del Paciente Crítico in Spanish).1 This document described the ethical and legal framework of end-of-life decisions, emphasized the need for integrating palliative care in the ICU setting and improving the skills for communicating bad news. Despite these recommendations are still valid and limitation of life-support treatment (LLST) strategies are a common practice in the ICU setting,2–4 the Bioethics WG decided to update them. The goal was to make them more widely known; reduce variability in the decision-making process5–7 and conflicts between parties; improve their documentation; and provide quality end-of-life care to families and patients. Since their publication, new LLST strategies have appeared like therapies that modify the course of diseases considered incurable a few years ago, but that also condition survival with sequelae or significant loss of quality of life. Also, the development of new less invasive life-support therapies in critically ill patients has promoted the patient autonomy. Also, organ donation has grown in situations different fron brain death by identifying patients who may die after making a LLST decision (called donation in controlled asystole). Quality improvement in the end-of-life care of critically ill patients has been an ongoing challenge for the SEMICYUC.8–10 Therefore, the Bioethics GW has designed this document and updated recommendations for the practice of LLST.
Work methodologyAt the SEMICYUC Bioethics WG meeting held in June 2017, it was agreed to update the recommendations on end-of-life care of critically ill patients1 following requests from this and other SEMICYUC WGs. Ten experts were selected based on their professional background and publications on bioethics. They discussed and chose the sections they would be developing, elaborated a chronogram, and assigned tasks. The WG coordinator and an expert on bioethics reviewed the contents and created the document writing group, AE and IS, adapting the style of the document text. Both the review and the recommendations were based on an updated search of biomedical literature on MEDLINE and EMBASE that analyzed clinical studies, systematic reviews and updates on end-of-life care and limitation of life-support treatment in adult critically ill patients. The document was assessed by 2 external reviewers (JLM and MR) and the document final version was sent to all authors and presented at the SEMICYUC Bioethics Working Group for its final approval.
End-of-life decision-making process in the critically ill patientLLST is a good medical practice because maintaining futile treatments and therapeutic obstinacy has no ethical or scientific justification.11 Wise decision-making should be based on a deliberation process of the actual facts and values. The inconsistency of clinical facts is often a source of conflict due to prognostic uncertainty and growing preoccupation on the sequelae derived from disease and treatments. In most cases, scientific evidence does not reach the necessary certainty to make proper clinical decisions per se. That is why the decision-making process should be based on the values and principles of the parties involved, especially the patient, and scientific evidence per se. Diagnosis and prognosis should also be refined and the entire process conducted by the entire healthcare team, not individually. The patient and, if possible, his relatives’ personal history of values and principles should be reviewed. Also, the existence of shared healthcare planning and advanced instruction documents when the patient does not have the capacity to make decisions by himself. The decision should be clinical and made by the healthcare team. Then the relatives should be informed of this decision in a way that does not add an extra burden of responsibility in these difficult times for them. If disagreement arises during the decision-making process, courses of action aimed at improving communication should be implemented. Also, reasonable periods of time to achieve some sort of understanding should be offered as well. Under no circumstance futile treatments required by the family should be administered or kept. The decisions made should be included in the patient’s clinical history. Quality palliative care should be provided12 favoring familial accompaniment and spiritual support.
If no consensus is reached among healthcare team, patient and relatives, the hospital Healthcare Ethics Committee may help. Fig. 1 shows a practical example of steps followed in the decision-making process of LLST strategies.
Shared healthcare planning and advanced instruction documentsShared healthcare planning (SHP) is a deliberative, relational and structured process to facilitate reflexion and understanding of the disease and care of the people involved. It focuses on the person who is going through the disease to identify and express his care preferences and expectations. Its goal is to promote shared decision-making processes when a person is not competent to decide by himself. It can include designating a representative, and the elaboration and registry of a document with values and preferences of treatment. Everything should be included in the patient’s clinical history for future reference of the team involved in the healthcare process. The denomination SHP includes the terms living will, advance care directives (documents with prior instructions [DPI] or advance directive forms), and advance care planning.13 Unlike other countries, in Spain SHP is only focused on document elaboration and registry. We should remember that personal reflexion and shared collaboration is the most important thing to improve the decision-making process, especially at the end of the patient’s life, when he can lose his ability to understand information and express his decision to accept or reject procedures and treatments.14
This patient-focused way of acting15 is not very common in our setting for various reasons:16 the little information healthcare providers have on SHP–including coping and communication skills of emotional aspects–; the lack of time needed to conduct in-depth interviews due to the healthcare load; the lack of specific SHP programs integrated in the healthcare routine or management of patients with chronic conditions; the perception from the healthcare providers that SHP only has to do with the process of dying and that it can emotionally damage the patients; lastly, the excessive bureaucracy associated with elaborating, registering, and consulting documents with prior instructions or advance directive forms.
Today the load of patient autonomy has gained specific weight in the daily clinical practice and it seems logical and necessary that he should be a part of his own care. Various studies show that the results of SHP improve the quality of end-of-life care.17–19Table 1 shows the advantages of SHP. Our daily practice in intensive care units tells us that few patients have documents with prior instructions prepared and when they do, they are in most cases unspecific and inappropriate for the intensive care setting.20 That is why advance directive forms expressing the patient’s will have become more and more important. To integrate SHP in the decision-making process of critically ill patients, our efforts should focus on the following aspects: popularize community programs including associations of patients by explaining how convenient it is to have a personal care and treatment plan; train healthcare providers to facilitate the elaboration, registry, and consultation of such plans; assess the impact SHP has on the decision-making process and on the quality of the end-of-life care provided to ICU patients.21,22 Back in 1994, the Hastings Center discussed the need to reorient DPI implementation strategies by not focusing on the documents but on the communicative processes among providers, patients, and relatives to improve the moral quality of these end-of-life decisions.23 This marked the beginning of advance planning with the following goals in mind: preparing oneself to die and all it implies; exercise the right to autonomy in the daily life; put this right into practice by expressing preferences of care and treatment; become familiar with the idea of dying; alleviate the emotional impact of the person designated by the patient to represent him when he cannot make any decisions by himself; understand the written and signed document as the conclusion of the planning process.24
Advantages of shared healthcare planning.
| • Promotes quality healthcare processes |
|---|
| • Establishes care and treatment goals |
| • Encourages the participation of patients and families in the decision-making process |
| • Reduces the number of unwanted treatments including hospital admissions and social and health spending without compromising the effectiveness and quality of healthcare |
| • Increases the satisfaction of citizens with the healthcare system |
| • Promotes healthy end-of-life decision-making processes and generates a greater social awareness on the importance of approaching death as a part of life |
| • Facilitates family mourning after the patient’s death |
LLST is defined as the omission or withdrawal of part or all life-support procedures like mechanical ventilation, hemofiltration, hemodialysis, and vasoactive medication. Over three decades ago, the Hasting Center defined life-support treatment as medical, technical, procedural or pharmacological interventions administered to a patient to delay the moment of death regardless of whether this treatment targets the underlying disease or actual biological process.
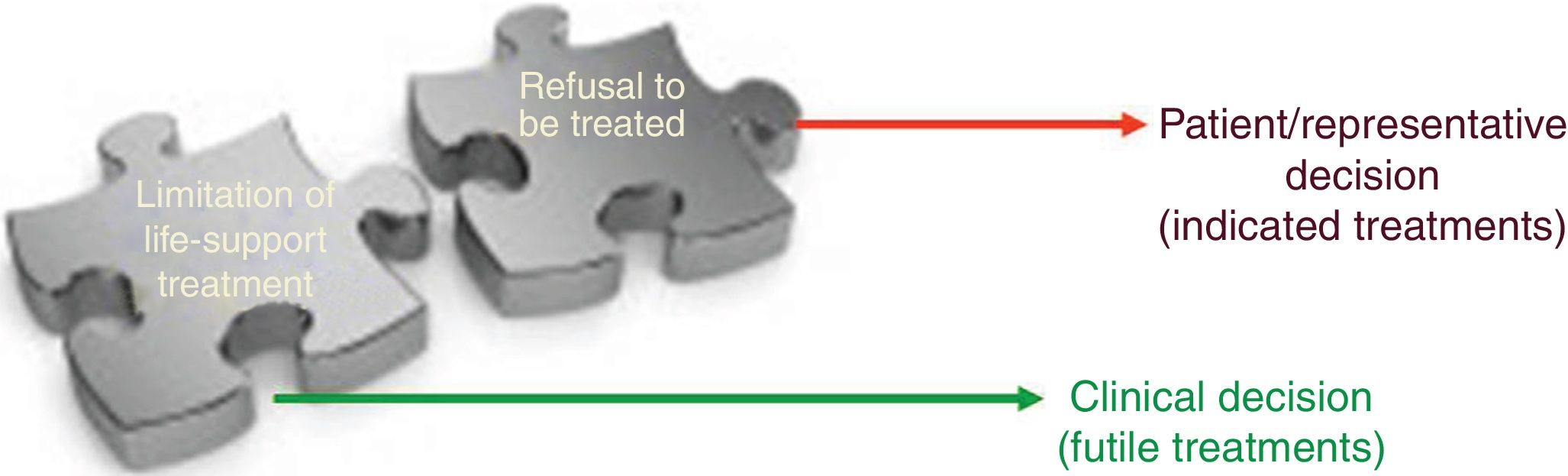
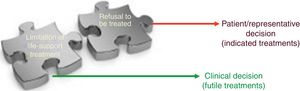
For healthcare providers LLSTs are clinical decisions and futility judgments consisting of weighed prognostic assessments on the ineffectiveness of a healthcare intervention. A procedure is considered futile when its application in a given clinical situation is ineffective and useless regarding the effect wanted. The main problem is the term futility—well-defined theoretically—but not so easy to identify in the clinical practice. Limitation of life-support treatment includes the decisions to omit and withdraw life-support treatment by healthcare providers on grounds of respect to the patient and his dignity. A different thing that does not come from the healthcare providers but from the patients themselves is refusal to receive treatment. With the actual ethical standards, promoting and protecting the patient autonomy makes him free to reject all sorts of life-support treatment even if prescribed by a doctor and even when the refusal can be life-treatening. Fig. 2 shows conceptual differences between refusing treatment and LLST.
Types of limitation of life-support treatmentTraditionally and arbitrarily the types of limitation of life-support treatment have been divided into 4 great categories: disregarding the patient admission to an ICU; omitting treatments; keeping therapies; withdrawing therapies.
The most common type of LLST is to omit treatment, being the order to refuse cardiopulmonary resuscitation the most common of all followed by the omission of preferably invasive treatments, and less frequently treatment withdrawal. Due to disease severity the mortality rate of treatment withdrawal is between 96% and 99%; treatment omission stays at 81%, and no further treatment, 44%.25 But there are new contributing factors in the decision-making process; the EPIPUSE study that analyzed LLST in Spanish ICUs in patients with long stays confirmed that in our country LLST happens 35% of the time with an associated hospital mortality rate of 93%. Also, this study confirmed that LLSTs are implemented in one third of the patients with complications or adverse events with organ repercussions that start during the second week of ICU admission.26 Although they are really well-categorized there is a huge variability in their application. A systematic review shows great differences in the ICUs of different countries, indicative of the existing needs for ethical training and research.27
New types of limitation of life-support treatment at the ICU settingToday more than 10% of all ICU admissions are patients > 80 years. This has led to changing LLST decisions that are more common in this type of patients.28 Changing the type of patients has increased the type of LLST at the moment of ICU admission.29 Mostly, the paradigm of LLST at ICU admission is geriatric, chronic complex, and oncological critically ill patients. This type of LLST at admission is rare (2%–8%) but still growing and the most interesting thing of all is that almost one third of the patients survive beyond the 30 day-threshold. This means that this type of limitation is not necessarily associated with an ominous prognosis. It consists of omitting mechanical ventilation, dialysis or CPR but not non-invasive treatments being treatment withdrawal extremely rare here.2
Another type of LLST is treatment withdrawal after treatment failure or clinical progression with poor prognosis. When making these decisions it is important to establish a time to reassess therapeutic response and explain the patient and/or his relatives the action plan to follow.30,31 For example, oncological patients who need mechanical ventilation and who, after predefining therapeutic goals at admission, are reevaluated a few days later to see if the treatment is working. In these cases, withdrawal decisions are made in case of irreversibility of the process.32 Other examples are LLSTs in patients with multi-organ failure in progress and SOFA > 15 or with severe brain damage and poor life expectancy.33
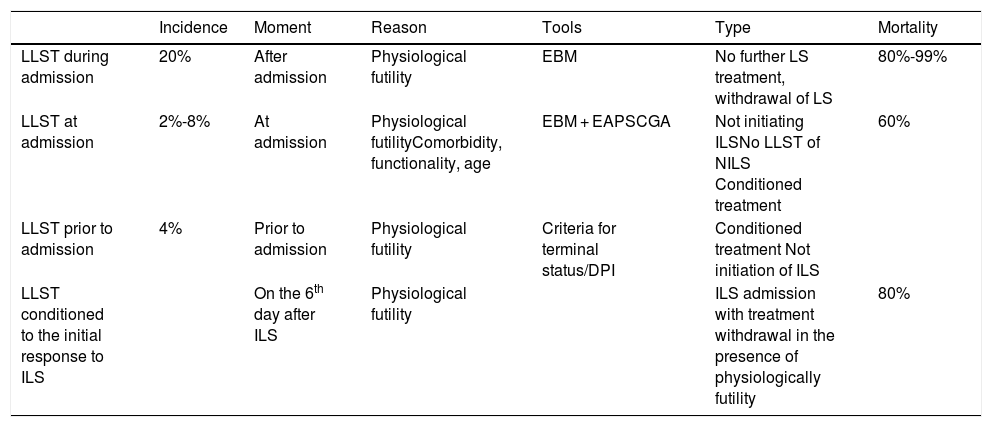
Table 2 shows the different types of LLST and the reason why they were implemented, the type of LLST implementation, and the mortality rate associated with each different type.
Different types of limitation of life-support treatments.
| Incidence | Moment | Reason | Tools | Type | Mortality | |
|---|---|---|---|---|---|---|
| LLST during admission | 20% | After admission | Physiological futility | EBM | No further LS treatment, withdrawal of LS | 80%-99% |
| LLST at admission | 2%-8% | At admission | Physiological futilityComorbidity, functionality, age | EBM + EAPSCGA | Not initiating ILSNo LLST of NILS Conditioned treatment | 60% |
| LLST prior to admission | 4% | Prior to admission | Physiological futility | Criteria for terminal status/DPI | Conditioned treatment Not initiation of ILS | |
| LLST conditioned to the initial response to ILS | On the 6th day after ILS | Physiological futility | ILS admission with treatment withdrawal in the presence of physiologically futility | 80% |
CGA, comprehensive geriatric assessment; DPI, document with prior instructions; EA, ethical analysis; EBM, evidence-based medicine; ILS, invasive life-support; LS, life support; NILS, non-invasive life support; PS, performance status.
There are different types of LLST based on the course of the disease, the moment of admission, and factors like patient’s frailty and disease-related life plan. Frailty can be a more solid predictor of vulnerability and recoverability than chronological age above all in the context of critical conditions.34,35 However, they are all LLSTs and their common ground is that certain treatments are not right for a patient at a given time due to being ineffective against an incurable disease, an advanced irreversible disease or due to the patient’s frailty. This makes certain treatments fail contrary to what would happen in other situations. Decisions to refuse ICU admission is another type of LLST that can be due to any of the considerations mentioned before. However, it is often implemented due to the latter after considering that ICU treatments may involve, in this particular situation, higher risk of complications and suffering compared to the benefits that could be expected.36 In these cases, the patients’ mortality rate is not 100% because prognosis does not have to be ominous at that time. However, if the patient deteriorates despite the treatment prescribed and requires major ICU life-support treatments, the complications associated with the patient’s frailty are expected to render the therapeutic escalation useless.
We need to make one consideration at this point. ICU admission does not necessarily mean new, more complex or high-risk treatments being prescribed. It may just involve surveillance and/or monitoring of the patient to optimize treatment without having to escalate to new therapies. If ICU admission can improve the patient’s prognosis it should not be rejected unless there are other causes.37 Age has been referred to as one of the numerous criteria to be considered when assessing the benefit of ICU admissions. Although it can be interrelated with others, age is not a criterion per se.38,39 Orders not to resuscitate can be agreed in patients admitted to the ICU, at the hospital floor or in other units. As we mentioned before, these patients’ mortality rate is not 100% either. That is so because these orders involve that if a cardiac arrest occurs it won’t be associated with an unexpected and potentially reversible situation but with the disease not responding to treatment. In this scenario, resustication maneuvers are not expected to succeed. The use of the hospital electronic health records can facilitate the decision-making process and management of these patients.40
Treatment omission/withdrawalThe WELPICUS study41 triggered international consensus to define the terms and concepts associated with end-of-life care at the ICU setting. The definitions are:
Life-support treatment omission: decision to not initiate life-support treatment. It may not be initiated in cases when a medical decision is made that a patient’s chances of survival are extremely low. Also, if in the actual medical circumstances, the patient does not want continued life-support.
Life-support treatment withdrawal: decision to actively stop an ongoing life-support treatment. It may be withdrawn in cases when a medical decision is made that a patient’s chances of survival are extremely low. Also, if in the actual medical circumstances, the patient does not want continued life-support.
LLST is not a reduced type of medical care but a restriction of measures considered disproportionate. Therefore, conservative and/or palliative care aimed at preserving the patient’s quality of life and alleviating pain or suffering should be continued.42,43
Proportionality of diagnostic methods and other treatments in end-of-life stages.
Decisions to refuse ICU admission or not resuscitate try to adequate treatment and diagnostic methods to the situation of each patient. When syndromic or etiological treatment proves useless, monitoring the symptoms, comfort, and family accompaniment should prevail. Although they should always be present in healthcare, in these cases they become priority goals. When healthcare providers, patients, and relatives are all aware of the irreversibility of a situation, priorities need to be established if they have not been agreed yet. Before initiating certain treatments or diagnostic methods to meet these priorities proper information needs to be provided in advance. Although LLST only refers to life-support treatments in certain clinical situations in the non-curative setting, this limitation may affect other treatments and diagnostic procedures that should be avoided unless they are measures to improve the patient’s symptoms.
Terminal extubationTerminal extubation (TE) is a type of withdrawing treatments previously started agreed with the patient or his representatives. It is performed in patients with failed treatments, in the absence of new and effective treatments, and when respiratory support is a disproportionate and senseless life-support treatment within the new therapeutic plan. In a high percentage of patients, TE should be performed in a relatively short period of time before the patient’s death due to disease progression. Death cannot be the direct cause of the treatments administered so TE should not be performed in patients with neuromuscular relaxation. These treatments should not be used so that the patient looks more placid after weaning from respiratory support. Muscle relaxants do not have sedative-analgesic effects and can make the examination of the patient difficult regarding pain or comfort. If the patient is already on these drugs, we should wait until the effect is gone. In some patients the lack of respiratory support may cause signs of respiratory failure44 due to an obstructed airway and dyspnea. These situations should be anticipated and avoided with the proper treatment like advanced sedation. That is why TE is often associated with some sort of analgesic-sedative treatment at adequate doses to avoid such symptoms. Depending on the cause of respiratory failure, oxygen, corticoids, diuretics, bronchodilators and/or opiods can be prescribed to treat the symptoms.45 Also, relatives46 and healthcare providers47 should be informed that the patient has developed these symptoms.
Planning clinical courses after LLSTAssociating LLST with death is a common mistake. The fact that most patients on LLSTs die due to the severe course of their disease does not mean that death will occur inexorably like former landmark studies have reported.48 One of the main goals here is to facilitate comfort, symptom control, and accompaniment of the loved ones. ICU humanization projects have tried to improve ICUs by relaxing the visiting hours and making the relatives part of the healthcare process.49 Therefore, it is essential to anticipate disease progression after the implementation of LLST decisions. Also, to contemplate action plans coordinated with other specialties like palliative care or hospital units to continue non-invasive treatments.
Palliative care in limitation of life-support treatmentWhen LLST has been decided, the healthcare plan should focus on avoiding suffering, respecting the patient’s dignity, and preventing or resolving the conflicts derived from the decision-making process. The comprehensive management of critically ill patients includes prevention, diagnosis, monitoring, and treatment of these patients plus proper palliative care (PC) after the LLST decision has been made.50
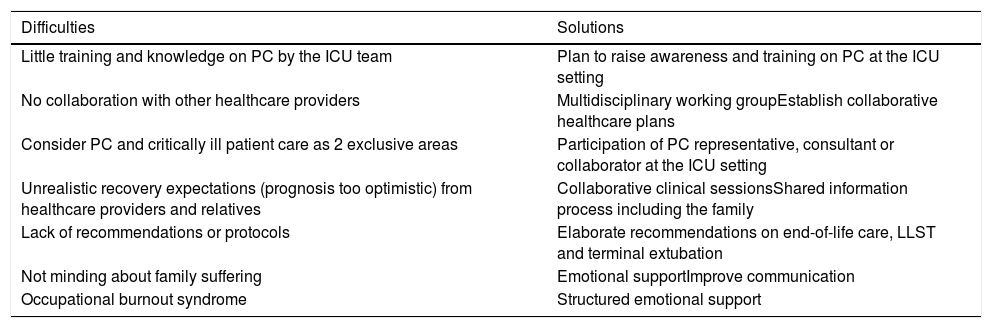
PC involves the administration of appropriate drugs to alleviate pain or dyspnea, family accompaniment, taking care of the patient’s needs, and facilitating a comfortable environment avoiding noises or unnecessary alarms. It consists of giving more importance gradually to care instead of treatment or diagnostic tests and making the relatives participate in that goal not only during the process of dying accompaniment but providing care during the process of mourning after the patient’s death. Table 3 shows the fundamental aspects of palliative care at the ICU setting. It is essential to plan LLSTs in advance, what treatments will be limited or withdrawn and how the events that may unfold and the presence of the family will be handled. Also, plan the duration of the visits; the number of people that will be accompanying the patient; inform the family of the different events that may occur like prolonged agony, survival of the patient, etc.; and teach them how to cope with them. Also, it is advisable to offer psychological support to the family. If the patient survives and is discharged from the ICU, it is advisable to promote the continuity of PC at the hospital floor unit with specific PC services only if appropriate and if prescribed by the healthcare team. By implementing comprehensive PC with the aspects mentioned above, the ICU can become a better place to die with personnel properly trained to treat the symptoms, accompany and comfort family and patient. It is a significant change that broadens the services provided in response to specific needs to ultimately improves the quality of end-of-life care. To achieve this we still have to break through SOME barriers; Table 4 shows the main difficulties and possible solutions for the integration of PC at the ICU setting.
Fundamental aspects of palliative care at the ICU setting.
| 1. Communicating the patient’s actual situation, prognosis and treatment options |
|---|
| 2. Establishing a healthcare and management plan taking the patient’s values and preferences into consideration |
| 3. Alleviating symptoms |
| 4. Providing emotional support to the relatives including care during mourning |
| 5. Care continuity |
| 6. Prevention and management of occupational burnout syndrome |
Difficulties and solutions to integrate palliative care at the ICU setting.
| Difficulties | Solutions |
|---|---|
| Little training and knowledge on PC by the ICU team | Plan to raise awareness and training on PC at the ICU setting |
| No collaboration with other healthcare providers | Multidisciplinary working groupEstablish collaborative healthcare plans |
| Consider PC and critically ill patient care as 2 exclusive areas | Participation of PC representative, consultant or collaborator at the ICU setting |
| Unrealistic recovery expectations (prognosis too optimistic) from healthcare providers and relatives | Collaborative clinical sessionsShared information process including the family |
| Lack of recommendations or protocols | Elaborate recommendations on end-of-life care, LLST and terminal extubation |
| Not minding about family suffering | Emotional supportImprove communication |
| Occupational burnout syndrome | Structured emotional support |
Intensive care to facilitate organ donation (ICOD) is the result of the changes made in the doctor-patient relation and affects 3 different dimensions: medical, ethical, and socicultural.50 ICOD should be included routinely in end-of-life care and are applicable to 2 different domains: the process of donation after brain death and the process of controlled/expected asystole donation. In both cases, the process of donation is built on a prior decision of limitation on criteria of futility often proposed by the treating physician and agreed with the patient and/or his relatives. Only when this decision has been made, the possibility of donation can be discussed. The identification and careful selection of patients eligible for ICOD is essential: patients with devastating irreversible neurological injuries with poor prognosis not eligible for ICU admission with curative purposes and who may evolve to brain death. Also, patients whose only reason for admission is being donors to avoid unnecessary or inappropriate admissions51 within a framework of excellent communication with the family and the medical teams.52 In patients who don’t meet the criteria for brain death, all procedures should be terminated. When appropriate, the possibility of asystole donation should be offered.
Expected asystole donation should be included as part of end-of-life care, once the LLST decision has been made, and in patients who may die after withdrawing life-support.
End-of-life communication with family and patientThe healthcare provider-patient relation is based on confidence and mutual sincerity. The patient is the actual holder of information and the only one entitled to decide whether to inform other people of his situation and status.53 A patient’s decision not to inform any third parties should be respected. Still, if appropriate, he should be persuaded that other people can participate in certain decisions not forseen in the instructions given by him when he cannot make any decisions by himself. However, in most cases, the status of the patient won’t let him participate in any end-of-life decisions at the ICU and relatives will become the main interlocutors unless the patient picked someone else.
There is an ongoing flow of information at the ICU not limited to a single day. In any case, if the course of the disease suggests that we are in an end-of-life situation, an interview will be necessary including a summary of what happened previously and what motivated the actual situation plus a treatment plan following the patient’s preferences. This information exchange should take place in an adequate setting.54 There is no unanimous criterion on what the best way to expose a patient’s diagnosis is. As a matter of fact, several studies warn that patients and relatives not always understand what doctors are trying to tell them. Several methods for decision-making or deliberating at the ICU setting have been suggested. Added to the healthcare team necessary consensus, we think LLST decisions should be shared with the patient and his relatives.55–60
Table 5 shows the recommendations established to make shared end-of-life healthcare decisions.61 The conflicts or discrepancies that may arise should be solved. Sometimes the family needs time to assimilate the information, postpone decisions, solve doubts or contact another physician who may have assisted the patient in the past and with whom patient and relatives have a relationship of trust. Also, the relatives need to be informed of the possible consequences of the actions that will unfold like breathing patterns prior to death that don’t necessarily mean suffering for the patient. Although the actual legislation and our own tradition consider the decisions made by relatives or legal representatives valid, several studies suggest that they do not reflect the actual wishes of the patient.62–64 We should explain and guide the relatives that they need to make a decision on behalf of the patient based on the decisions he would have made by himself. The information and the decisions made in these interviews should be included in the patient’s clinical history. No written informed consent is required for these proceedings that are nothing but an adjustment of treatment. Telling bad news and making end-of-life decisions is something intensivists and other healthcare providers involved in the management of critically ill patients should learn and train.65 The possibility of providing spiritual support to patients and relatives should be explored too; most families are thankful when offered even if they did not ask or want it.66
Procedure for shared end-of-life healthcare decisions at the ICU setting.
| 1. Talk about the nature of the decision that needs to be made |
|---|
| 2. Describe treatment options |
| 3. Talk about the pros and the cons of the treatment options available |
| 4. Comment on the uncertainty of each case |
| 5. Verify whether the relatives have understood the information provided |
| 6. Obtain information on the patient’s values and preferences |
| 7. Validate the representatives’ preferences on their role in the decision-making process |
| 8. Assess whether participation of other people is required |
| 9. Take the consequences of the decision made into consideration |
| 10. Ask the representatives what they think of the decision suggested |
Measuring quality is essential to guarantee and improve end-of-life care in the critically ill patient. The key domains of high-quality palliative care have been defined by patients, relatives,67 and expert consensus.60,68 These domains include the effective management of physical, psychological, and spiritual symptoms, and the timely and sensitive communication on the adequacy of care in relation to the situation of the patient, prognosis, and values. Also, match treatment to the patient’s preferences, care for the family needs, plan care transitions, and support to healthcare providers. Quality of end-of-life care has also been assessed through surveys including providers, both doctors and nurses, and relatives and validated in the United States69 and, recently, Europe.70 Several studies have reported the margin for improvement of many ICUs on a global scale.71
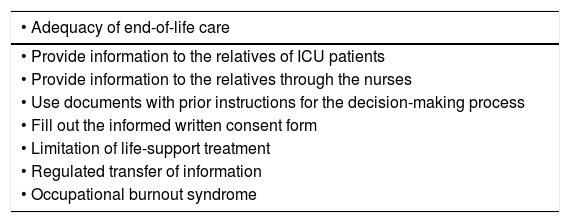
Quality assessment can be performed through indicators of structure, process and result.10,72 Most proposals describe process indicators, but there is less consensus on result indicators.73 Also, we need studies on the process-results correlation guaranteeing the effectiveness, efficiency, and viability of the different interventions proposed over the last few years to improve the quality of end-of-life care. Also, we need to know what interventions can lead to unwanted consequences.74,75 Ever since 2005, SEMICYUC has been elaborating quality indicators—recently updated—including a significant number of end-of-life care indicators 76 as shown on Table 6. Measuring these indicators periodically allows us to identify situations of improvement, compare results, and establish better practices to guarantee excellent end-of-life care.
SEMICYUC quality indicators associated with end-of-life care.
| • Adequacy of end-of-life care |
|---|
| • Provide information to the relatives of ICU patients |
| • Provide information to the relatives through the nurses |
| • Use documents with prior instructions for the decision-making process |
| • Fill out the informed written consent form |
| • Limitation of life-support treatment |
| • Regulated transfer of information |
| • Occupational burnout syndrome |
Healthcare providers can suffer from stress after being involved in end-of-life situations; ethical conflicts; constantly seeing the patients’ suffering; perceiving potentially inappropriate or futile care; inadequately communicating with the team; and having to care for the family needs. On many occasions, healthcare providers have to face these situations without the proper training in clinical ethics. These work-related stress factors can trigger specific syndromes like moral suffering, perception of inappropriate care, compassion-induced fatigue, secondary traumatic stress, and occupational burnout, among others.77 On many occasions, these syndromes are closely related to one another and overlap in some professionals. They can have a negative personal and professional impact and repercussions in the quality of care provided to patients and relatives, favor professional absenteeism, and increase costs.78
To have a healthy working space we need multimodal interventions aimed at improving communication skills, collaboration, effective decision-making processes, adequate ratios of professionals, personal recognition, and real leadership. Training in bioethics and interdisciplinary debates during and after conflicting situations may reduce the appearance of these symptoms.79 Healthcare providers need to be aware of risky situations and ask for help when needed. Similarly, individual strategies should focus on promoting the healthcare providers’ physical and emotional self-esteem, and resilience.
Final considerationsThe valuable contribution of bioethics to intensive medicine is the introduction of values in the complex decision-making process to improve these decisions. LLST means omitting or withdrawing futile treatments at a certain point after confirming the mismatch between the therapeutic means and their goals. Today, LLST is a common clinical practice at the ICU setting. Fighting therapeutic obstinacy is a primary goal of end-of-life care. The recommendations established by SEMICYUC Bioethics Working Group emphasize the importance of shared healthcare planning including donation-oriented intensive care, and new types of LLST. At the same time, they promote integrating palliative care in the ICU setting and improving communication and family accompaniment plans. These recommendations have been elaborated by SEMICYUC Bioethics Working Group to reduce the variability of clinical practice observed in LLST stratgies and contribute to improve the end-of-life care of critically ill patients.
Conflicts of interestNone declared.
Please cite this article as: Estella Á, Saralegui I, Rubio Sanchiz O, Hernández-Tejedor A, López Camps V, Martín MC, et al. Puesta al día y recomendaciones en la toma de decisiones de limitación de tratamientos de soporte vital. Med Intensiva. 2020;44:101–112.