To describe trends in national catheter-related urinary tract infection (CRUTI) rates, as well as etiologies and multiresistance markers.
DesignAn observational, prospective, multicenter voluntary participation study was conducted from 1 April to 30 June in the period between 2005 and 2010.
SettingIntensive Care Units (ICUs) that participated in the ENVIN-ICU registry during the study period.
PatientsWe included all patients admitted to the participating ICUs and patients with urinary catheter placement for more than 24h (78,863 patients).
InterventionPatient monitoring was continued until discharge from the ICU or up to 60 days.
Variables of interestCRUTIs were defined according to the CDC system, and frequency is expressed as incidence density (ID) in relation to the number of urinary catheter-patients days.
ResultsA total of 2329 patients (2.95%) developed one or more CRUTI. The ID decreased from 6.69 to 4.18 episodes per 1000 days of urinary catheter between 2005 and 2010 (p<0.001). In relation to the underlying etiology, gramnegative bacilli predominated (55.6–61.6%), followed by fungi (18.7–25.2%) and grampositive cocci (17.1–25.9%). In 2010, ciprofloxacin-resistant Escherichia coli strains (37.1%) increased, as well as imipenem-resistant (36.4%) and ciprofloxacin-resistant (37.1%) strains of Pseudomonas aeruginosa.
ConclusionsA decrease was observed in CRUTI rates, maintaining the same etiological distribution and showing increased resistances in gramnegative pathogens, especially E. coli and P. aeruginosa.
Describir la evolución de las tasas nacionales de las infecciones urinarias relacionada con sonda uretral (IU-SU), así como la de sus etiologías y marcadores de multirresistencia.
DiseñoEstudio observacional, prospectivo, de participación voluntaria y multicéntrico desde el 1 de abril al 30 de junio entre los años 2005 y 2010.
ÁmbitoUnidades de Cuidados Intensivos (UCI) que participaron en el registro ENVIN-UCI en el periodo de estudio.
PacientesSe han incluido todos los pacientes ingresados en las UCI participantes y portadores de sonda urinaria durante más de 24 horas (78.863 pacientes).
IntervenciónLa vigilancia de los pacientes ha sido continua hasta el alta de UCI o un máximo de 60 días.
Variables de interésSe han definido las IU-SU siguiendo los criterios del CDC y su frecuencia se expresa como densidad de incidencia (DI) en relación al número de días de paciente-SU.
ResultadosHan presentado una o más IU-SU 2.329 (2,95%) pacientes. La DI de IU-SU ha disminuido desde 6,69 a 4,18 episodios por 1.000 días de SU desde el año 2005 al año 2010 (p<0,001). En la etiología han predominado los bacilos gramnegativos (55,6-61,6%), seguido de hongos (18,7-25,2) y de los cocos grampositivos (17,1-25,9%). En el año 2010, han aumentado las cepas de E. coli resistentes a ciprofloxacino (37,1%) y de P. aeruginosa resistente a imipenem (36,4%) y ciprofloxacino (37,1%).
ConclusionesDisminución de las tasas de IU-SU, manteniéndose la misma distribución de etiología e incrementándose las resistencia en los BGN, en especial el Escherichia coli y Pseudomonas aeruginosa.
Urethral catheter-related urinary tract infection (CRUTI) is one of the most frequent infections in patients admitted to the Intensive Care Unit (ICU), after ventilator associated pneumonia (VAP) and primary or vascular catheter-related bacteremia.1,2 In most cases the diagnosis is based on the isolation of a significant number of bacteria or fungi per milliliter of urine in patients with clinical evidence of systemic inflammatory response. The presence of signs or symptoms specific of CRUTI is less common.
The etiology of CRUTI in critical patients admitted to the ICU presents some differences with respect to the urinary infections seen in the rest of the patients admitted to hospital, due to the presence of certain pathogens both in the patient and in the surroundings, selected as a result of frequent antibiotic use.3 The increase in resistances on the part of these pathogens can condition changes in the empirical treatment of these infections. Nevertheless, the information referred to this infectious problem in critical patients is limited.
For years, the Infectious Diseases Working Group of the Spanish Society of Intensive and Critical Care Medicine and Coronary Units (Grupo de Trabajo de Enfermedades Infecciosas de la Sociedad Española de Medicina Intensiva, Crítica y Unidades Coronarias, GTEI-SEMICYUC) has promoted the vigilance of infections acquired in the ICU and related to the use of medical devices. The National Study for the Vigilance of Nosocomial Infections in the ICU (ENVIN-ICU) has registered infections of this kind in Spanish ICUs since 1994.4 The results referred to the evolution of the CRUTI rates, the underlying etiologies, and the multiple resistance markers of the most common pathogens are described in the present study.
Patients and methodsStudy designA prospective, multicenter, voluntary participation, observational study was carried out.
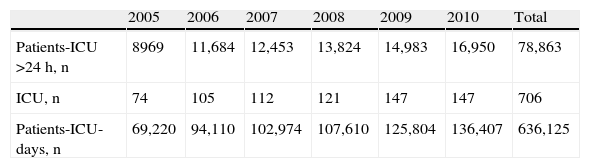
PatientsThe study prospectively included all patients with a urinary catheter in place for over 24h, admitted to the participating ICUs between 1 April and 31 June corresponding to the period 2005–2010 (both included). The number of patients added to the registry each year, the participating ICUs, and the total number of patients-day in the ICU are shown in Table 1.
Number of patients, participating ICUs and patients-day admitted in each of the years of the registry.
| 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | Total | |
| Patients-ICU >24h, n | 8969 | 11,684 | 12,453 | 13,824 | 14,983 | 16,950 | 78,863 |
| ICU, n | 74 | 105 | 112 | 121 | 147 | 147 | 706 |
| Patients-ICU-days, n | 69,220 | 94,110 | 102,974 | 107,610 | 125,804 | 136,407 | 636,125 |
The patients admitted before the first day of the control period and who remained in the Unit during the study phase were not subjected to follow-up. Monitoring of the patients included in the study was continuous until discharge from the ICU or for a maximum of 60 days.
The following information was collected in all patients: demographic data, background disease, instrumentation performed (intubation and mechanical ventilation, urethral catheterization, central venous catheters and arterial catheters), hospital stay prior to admission to the ICU, stay in the ICU, and clinical condition at the time of discharge from the ICU. The patients were classified according to reason for admission: “coronary” when the reason for admission was ischemic coronary or heart disease; “trauma” when the reason for admission was injury affecting any body location; “surgical” when the patients were admitted in the immediate postoperative period of elective surgery; and “clinical” in the rest of the cases.
Patient severity was assessed by the APACHE II score.5 The need for emergency surgery was defined as the need for non-elective surgery before or during the stay in the ICU. Raw mortality was defined as mortality in the ICU due to any reason.
Diagnosis of CRUTIThe criteria used for defining CRUTI have been published in the manual of the ENVIN project,6 following the indications of the United States Centers for Disease Control (CDC).7 Diagnosis required the clinical and/or microbiological signs to be absent during the incubation period and at the time of urethral catheterization. The patients were required to have one of the following three clinical signs and/or symptoms: fever >38°C, tension in the suprapubic zone, or micturition urgency and pyuria. The latter was defined as over 10 leukocytes/ml of urine or more than three leukocytes/ml in a non-centrifuged sample. Likewise, the patients were required to meet one of the following two microbiological criteria: (a) in subjects without antibiotics, urine culture with the isolation of ≥105cfu/ml of no more than two microorganisms; and (b) in patients with antibiotics, urine culture with <105cfu/ml of a single microorganism.
CRUTI was diagnosed by the physicians in charge of the patients, and was documented as such in the case history. Those in charge of the vigilance of nosocomial infections were intensivists with special interest and training in infectious disease processes. These physicians prospectively registered the infections, but did not directly intervene in their diagnosis.
Systemic response to CRUTI was assessed as sepsis, severe sepsis or septic shock, following the criteria of the 2001 consensus conference.8
Regarding the etiological diagnosis of CRUTI, we accepted the procedures followed by the laboratories of the participating hospital centers. Susceptibility to the different antibiotics on the part of the pathogens identified as causing the infections was determined following the specifications (method and values) of the National Committee for Clinical Laboratory Standards (NCCLS, 1995)9 of the Clinical and Laboratory Standards Institute (CLSI),10 and more recently of the European Committee on Antimicrobial Susceptibility Testing (EUCAST).11 Resistance markers of the most frequent pathogens have been defined: in Escherichia coli (E. coli) and Proteus mirabilis (P. mirabilis), cefotaxime and ciprofloxacin; in Pseudomonas aeruginosa (P. aeruginosa), imipenem, ciprofloxacin and amikacin; in Enterococcus faecalis (E. faecalis), vancomycin and ampicillin; and in Candida albicans (C. albicans), fluconazole.
Risk factorsThe risk factors were calculated globally for all the patients admitted during the vigilance period, following the criteria of the National Nosocomial Infections Surveillance (NNIS).12 To this effect we recorded the patients with an urethral catheter on a daily basis. Calculation was made of the urethral catheter utilization ratio, defined as the ratio between the number of days of utilization of the urethral catheter and the days of risk (days of stay).
Incidence measuresCalculation was made of the cumulative incidence (CI) and the incidence density (ID). The cumulative incidence was calculated by dividing the number of CRUTI episodes by the total patients included in the vigilance period, expressed as a percentage. The incidence density in turn was calculated by dividing the number of CRUTI episodes by the total days of urethral catheterization exposure. This parameter was expressed as the number of CRUTI episodes per 1000 days of urethral catheterization exposure.
Statistical analysisData collection was carried out using the ENVIN-ICU software application, located in a web server, and which was accessed online. The database is located in the same server (SQL Server). The program is equipped with security systems requiring entry of the variables defined as being basic, and precluding the entry of illogical values. Qualitative variables were reported with the distribution percentage of each of the categories. Quantitative variables in turn were reported with the mean and standard deviation in the case of a normal distribution, and with the median and interquartile range in the case of a non-normal distribution. Comparison of the incidence of CRUTI according to the different qualitative variables (e.g., background disease or the presence of emergency surgery) was based on the chi-squared test (χ2). In the case of ordinal variables (categorized APACHE II or age), the chi-squared test (χ2) for linear tendencies was used. The accepted level of statistical significance was 5% (p<0.05).
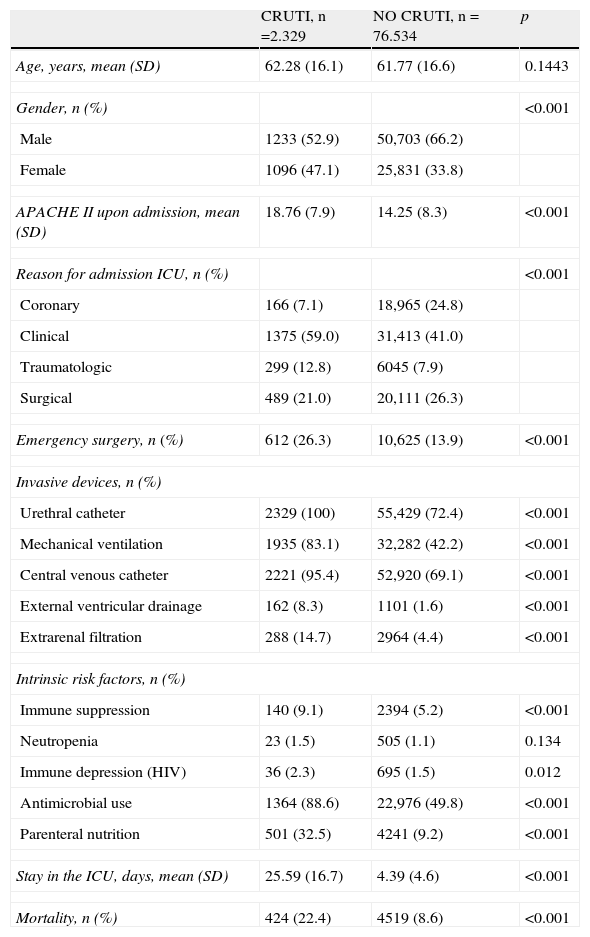
ResultsDuring the 6 years analyzed, a total of 78,863 patients were included, of which 2329 (2.95%) suffered one or more CRUTI episodes during admission to the ICU. The characteristics of the patients according to the presence or absence of CRUTI are shown in Table 2. The presence of such infection was frequent in women, in the more seriously ill patients upon admission, and in the subjects classified as corresponding to clinical or surgical cases. Likewise, CRUTI predominated among patients with invasive devices, replacement therapies (parenteral nutrition, extrarenal filtration) and in those with risk factors related to their disease (neutropenia, immune suppression and immune depression–the latter referring to acquired depression and HIV infection).
Characteristics and evolution of the patients according to the presence or absence of CRUTI.
| CRUTI, n=2.329 | NO CRUTI, n=76.534 | p | |
| Age, years, mean (SD) | 62.28 (16.1) | 61.77 (16.6) | 0.1443 |
| Gender, n (%) | <0.001 | ||
| Male | 1233 (52.9) | 50,703 (66.2) | |
| Female | 1096 (47.1) | 25,831 (33.8) | |
| APACHE II upon admission, mean (SD) | 18.76 (7.9) | 14.25 (8.3) | <0.001 |
| Reason for admission ICU, n (%) | <0.001 | ||
| Coronary | 166 (7.1) | 18,965 (24.8) | |
| Clinical | 1375 (59.0) | 31,413 (41.0) | |
| Traumatologic | 299 (12.8) | 6045 (7.9) | |
| Surgical | 489 (21.0) | 20,111 (26.3) | |
| Emergency surgery, n (%) | 612 (26.3) | 10,625 (13.9) | <0.001 |
| Invasive devices, n (%) | |||
| Urethral catheter | 2329 (100) | 55,429 (72.4) | <0.001 |
| Mechanical ventilation | 1935 (83.1) | 32,282 (42.2) | <0.001 |
| Central venous catheter | 2221 (95.4) | 52,920 (69.1) | <0.001 |
| External ventricular drainage | 162 (8.3) | 1101 (1.6) | <0.001 |
| Extrarenal filtration | 288 (14.7) | 2964 (4.4) | <0.001 |
| Intrinsic risk factors, n (%) | |||
| Immune suppression | 140 (9.1) | 2394 (5.2) | <0.001 |
| Neutropenia | 23 (1.5) | 505 (1.1) | 0.134 |
| Immune depression (HIV) | 36 (2.3) | 695 (1.5) | 0.012 |
| Antimicrobial use | 1364 (88.6) | 22,976 (49.8) | <0.001 |
| Parenteral nutrition | 501 (32.5) | 4241 (9.2) | <0.001 |
| Stay in the ICU, days, mean (SD) | 25.59 (16.7) | 4.39 (4.6) | <0.001 |
| Mortality, n (%) | 424 (22.4) | 4519 (8.6) | <0.001 |
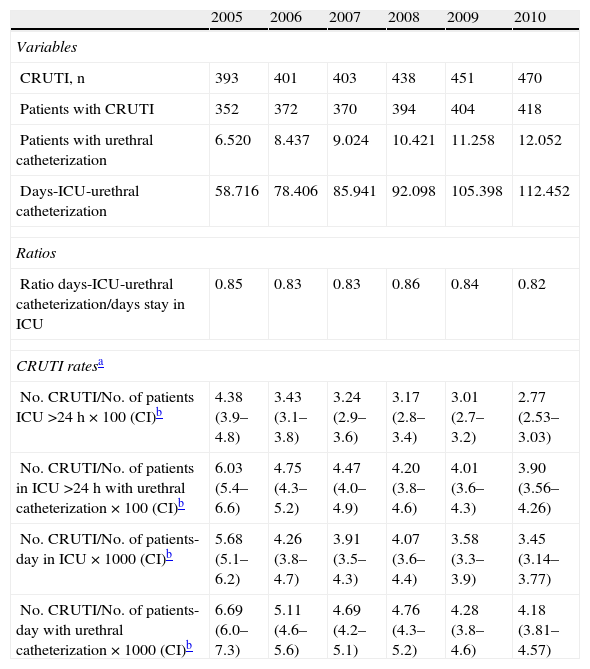
Table 3 includes the number of CRUTI episodes detected in each of the years of the study, along with the number of patients in which these infections were identified, the number of patients with urethral catheterization, and the total patients-day with urethral catheterization. Based on these variables, calculation was made of the four ways of expressing the frequency of these infections. The ratio between the number of days corresponding to the patients admitted to the ICU with urethral catheterization and the total number of days corresponding to the patients admitted to the ICU remained constant over time, in the order of 0.85 (95%CI: 0.83–0.86), while the different CRUTI incidence indicators decreased significantly (p<0.001) (Table 3).
Evolution of the CRUTI frequency indicators during the study period.
| 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | |
| Variables | ||||||
| CRUTI, n | 393 | 401 | 403 | 438 | 451 | 470 |
| Patients with CRUTI | 352 | 372 | 370 | 394 | 404 | 418 |
| Patients with urethral catheterization | 6.520 | 8.437 | 9.024 | 10.421 | 11.258 | 12.052 |
| Days-ICU-urethral catheterization | 58.716 | 78.406 | 85.941 | 92.098 | 105.398 | 112.452 |
| Ratios | ||||||
| Ratio days-ICU-urethral catheterization/days stay in ICU | 0.85 | 0.83 | 0.83 | 0.86 | 0.84 | 0.82 |
| CRUTI ratesa | ||||||
| No. CRUTI/No. of patients ICU >24h×100 (CI)b | 4.38 (3.9–4.8) | 3.43 (3.1–3.8) | 3.24 (2.9–3.6) | 3.17 (2.8–3.4) | 3.01 (2.7–3.2) | 2.77 (2.53–3.03) |
| No. CRUTI/No. of patients in ICU >24h with urethral catheterization×100 (CI)b | 6.03 (5.4–6.6) | 4.75 (4.3–5.2) | 4.47 (4.0–4.9) | 4.20 (3.8–4.6) | 4.01 (3.6–4.3) | 3.90 (3.56–4.26) |
| No. CRUTI/No. of patients-day in ICU×1000 (CI)b | 5.68 (5.1–6.2) | 4.26 (3.8–4.7) | 3.91 (3.5–4.3) | 4.07 (3.6–4.4) | 3.58 (3.3–3.9) | 3.45 (3.14–3.77) |
| No. CRUTI/No. of patients-day with urethral catheterization×1000 (CI)b | 6.69 (6.0–7.3) | 5.11 (4.6–5.6) | 4.69 (4.2–5.1) | 4.76 (4.3–5.2) | 4.28 (3.8–4.6) | 4.18 (3.81–4.57) |
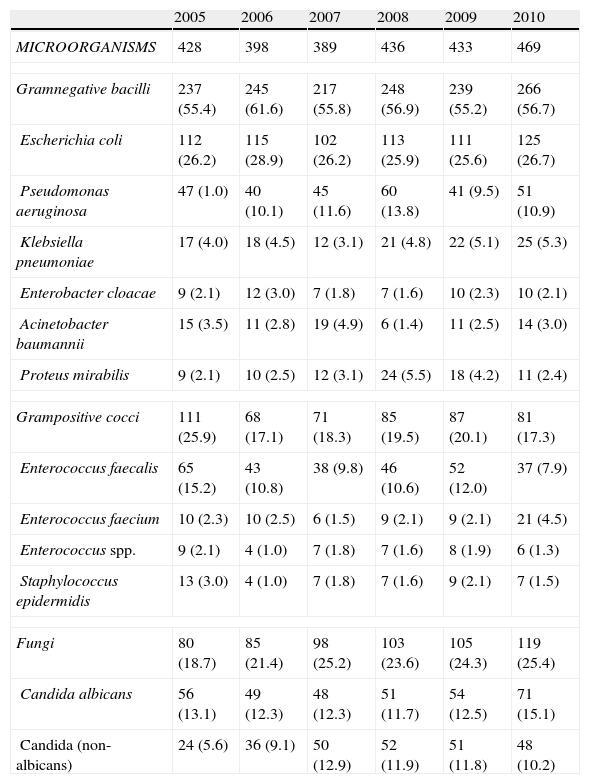
Table 4 shows the etiology of all the CRUTI episodes. Gramnegative bacilli were seen to predominate during the analyzed time period, representing between 55 and 60% of all isolates, and with a particularly important presence of E. coli. This was followed by fungi (19–25% of all isolates), all pertaining to the genus Candida, where C. albicans was identified in one-half of the episodes. Among the grampositive cocci, enterococci were seen to predominate (particularly E. faecalis).
Evolution of the etiology of CRUTI (n and % with respect to total microorganisms).
| 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | |
| MICROORGANISMS | 428 | 398 | 389 | 436 | 433 | 469 |
| Gramnegative bacilli | 237 (55.4) | 245 (61.6) | 217 (55.8) | 248 (56.9) | 239 (55.2) | 266 (56.7) |
| Escherichia coli | 112 (26.2) | 115 (28.9) | 102 (26.2) | 113 (25.9) | 111 (25.6) | 125 (26.7) |
| Pseudomonas aeruginosa | 47 (1.0) | 40 (10.1) | 45 (11.6) | 60 (13.8) | 41 (9.5) | 51 (10.9) |
| Klebsiella pneumoniae | 17 (4.0) | 18 (4.5) | 12 (3.1) | 21 (4.8) | 22 (5.1) | 25 (5.3) |
| Enterobacter cloacae | 9 (2.1) | 12 (3.0) | 7 (1.8) | 7 (1.6) | 10 (2.3) | 10 (2.1) |
| Acinetobacter baumannii | 15 (3.5) | 11 (2.8) | 19 (4.9) | 6 (1.4) | 11 (2.5) | 14 (3.0) |
| Proteus mirabilis | 9 (2.1) | 10 (2.5) | 12 (3.1) | 24 (5.5) | 18 (4.2) | 11 (2.4) |
| Grampositive cocci | 111 (25.9) | 68 (17.1) | 71 (18.3) | 85 (19.5) | 87 (20.1) | 81 (17.3) |
| Enterococcus faecalis | 65 (15.2) | 43 (10.8) | 38 (9.8) | 46 (10.6) | 52 (12.0) | 37 (7.9) |
| Enterococcus faecium | 10 (2.3) | 10 (2.5) | 6 (1.5) | 9 (2.1) | 9 (2.1) | 21 (4.5) |
| Enterococcus spp. | 9 (2.1) | 4 (1.0) | 7 (1.8) | 7 (1.6) | 8 (1.9) | 6 (1.3) |
| Staphylococcus epidermidis | 13 (3.0) | 4 (1.0) | 7 (1.8) | 7 (1.6) | 9 (2.1) | 7 (1.5) |
| Fungi | 80 (18.7) | 85 (21.4) | 98 (25.2) | 103 (23.6) | 105 (24.3) | 119 (25.4) |
| Candida albicans | 56 (13.1) | 49 (12.3) | 48 (12.3) | 51 (11.7) | 54 (12.5) | 71 (15.1) |
| Candida (non-albicans) | 24 (5.6) | 36 (9.1) | 50 (12.9) | 52 (11.9) | 51 (11.8) | 48 (10.2) |
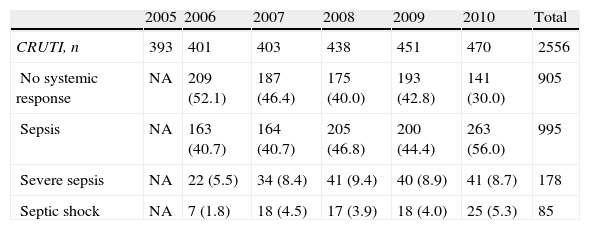
The systemic response associated to CRUTI is reported in Table 5. In one-half of the cases there was no systemic inflammatory response, and in less than 15% of the cases the response was classified as serious.
Systemic response of the patients with CRUTI (n, %).
| 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | Total | |
| CRUTI, n | 393 | 401 | 403 | 438 | 451 | 470 | 2556 |
| No systemic response | NA | 209 (52.1) | 187 (46.4) | 175 (40.0) | 193 (42.8) | 141 (30.0) | 905 |
| Sepsis | NA | 163 (40.7) | 164 (40.7) | 205 (46.8) | 200 (44.4) | 263 (56.0) | 995 |
| Severe sepsis | NA | 22 (5.5) | 34 (8.4) | 41 (9.4) | 40 (8.9) | 41 (8.7) | 178 |
| Septic shock | NA | 7 (1.8) | 18 (4.5) | 17 (3.9) | 18 (4.0) | 25 (5.3) | 85 |
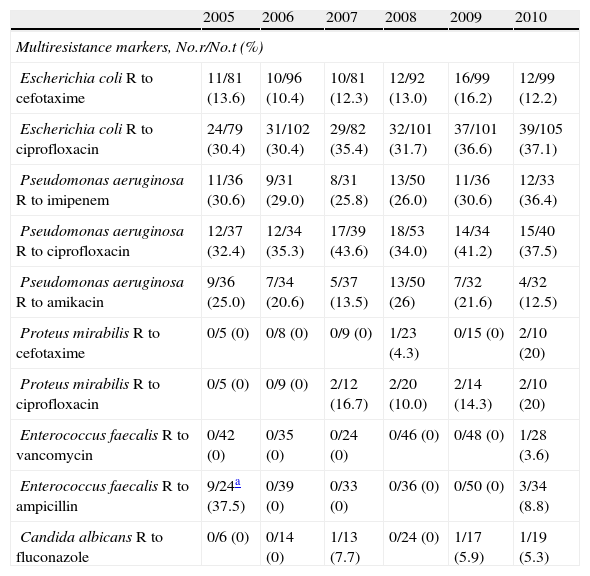
The multiresistance markers of the main pathogens causing CRUTI showed important differences (Table 6). While in the case of E. coli we observed a progressive increase in strains resistant to ciprofloxacin, no such increase was observed in relation to cefotaxime. In the case of P. aeruginosa, resistance to ciprofloxacin and imipenem exceeded 30%, while resistance to ampicillin and vancomycin was practically inexistent in E. faecalis. Likewise, no C. albicans strains were found to be resistant to fluconazole.
Evolution of the multiresistance markers of the most common pathogens in CRUTI.
| 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | |
| Multiresistance markers, No.r/No.t (%) | ||||||
| Escherichia coli R to cefotaxime | 11/81 (13.6) | 10/96 (10.4) | 10/81 (12.3) | 12/92 (13.0) | 16/99 (16.2) | 12/99 (12.2) |
| Escherichia coli R to ciprofloxacin | 24/79 (30.4) | 31/102 (30.4) | 29/82 (35.4) | 32/101 (31.7) | 37/101 (36.6) | 39/105 (37.1) |
| Pseudomonas aeruginosa R to imipenem | 11/36 (30.6) | 9/31 (29.0) | 8/31 (25.8) | 13/50 (26.0) | 11/36 (30.6) | 12/33 (36.4) |
| Pseudomonas aeruginosa R to ciprofloxacin | 12/37 (32.4) | 12/34 (35.3) | 17/39 (43.6) | 18/53 (34.0) | 14/34 (41.2) | 15/40 (37.5) |
| Pseudomonas aeruginosa R to amikacin | 9/36 (25.0) | 7/34 (20.6) | 5/37 (13.5) | 13/50 (26) | 7/32 (21.6) | 4/32 (12.5) |
| Proteus mirabilis R to cefotaxime | 0/5 (0) | 0/8 (0) | 0/9 (0) | 1/23 (4.3) | 0/15 (0) | 2/10 (20) |
| Proteus mirabilis R to ciprofloxacin | 0/5 (0) | 0/9 (0) | 2/12 (16.7) | 2/20 (10.0) | 2/14 (14.3) | 2/10 (20) |
| Enterococcus faecalis R to vancomycin | 0/42 (0) | 0/35 (0) | 0/24 (0) | 0/46 (0) | 0/48 (0) | 1/28 (3.6) |
| Enterococcus faecalis R to ampicillin | 9/24a (37.5) | 0/39 (0) | 0/33 (0) | 0/36 (0) | 0/50 (0) | 3/34 (8.8) |
| Candida albicans R to fluconazole | 0/6 (0) | 0/14 (0) | 1/13 (7.7) | 0/24 (0) | 1/17 (5.9) | 1/19 (5.3) |
No.r: number of strains resistant to the marker antibiotic; No.t: total strains for which resistance information has been supplied.
The patients with CRUTI had a longer stay in the ICU (25.59 versus 4.39 days in those without CRUTI) and greater global mortality in the ICU (22.4% versus 8.6%)–though the available data do not allow us to establish a relationship between these variables and the presence of CRUTI.
DiscussionThe main contribution of this study has been the description of the CRUTI rates, etiologies and multiresistance markers in critical patients admitted to the ICU in the last 6 years (2005–2010). The evolutive data reflect a significant decrease in CRUTI, independent of the rate used to express the infections. In the last year of the study, the rate expressed as incidence density (ID) was 4.18 episodes per 1000 days of urethral catheterization. This is considered to be within the limits published by the National Healthcare Safety Network (NHSN) in December 2009 with the cumulative data corresponding to the period between 2006 and 2008 in American ICUs.13 The ID of CRUTI varied between 7.4 episodes per 1000 days of urethral catheterization in the ICU for burn or neurological patients and 3.1 episodes per 1000 days of urethral catheterization in clinical-surgical ICUs with more than 15 beds–the national average calculated from the data being 4.08 episodes per 1000 days of urethral catheterization. The references to CRUTI in the literature range from 1.4 episodes per 1000 days of urethral catheterization in 12 ICUs in India14 to 5.1 episodes per 1000 days of urethral catheterization in four Peruvian ICUs,15 while the most recently published data report 15.7 episodes per 1000 days of urethral catheterization in four ICUs in Alexandria (Egypt).16
The CRUTI rates in Spanish ICUs have decreased significantly in recent years, without the application of any specific national project or initiative for the prevention of these infections. During this period of time there have been specific campaigns aiming to promote the washing of hands among healthcare workers in most Spanish hospitals.17 Furthermore, a national campaign has been started for preventing catheter-related bacteremias in the ICU (the bacteremia zero project), based on the introduction of a package of measures (one of which consists of washing of the hands before and after manipulating the catheter),18 and the concept of integral patient safety has been divulgated through courses and practical cases. Globally, these activities designed to promote a safe environment in the ICU may have contributed to improve the CRUTI rates.The etiology of CRUTI has remained without change during these years, being headed by gramnegative bacilli (especially E. coli), followed by other microorganisms such as E. faecalis and C. albicans.19 Other pathogens that may be endemic to ICUs and remain in the environment are much less frequent, including methicillin-resistant S. aureus (MRSA), P. aeruginosa and Acinetobacter baumannii (A. baumannii), which are the main causes of other nosocomial infections such as ventilator associated pneumonia (VAP).20 This underscores the importance of the intestinal flora in the etiology of these infections and the need to maximize all measures that limit or complicate the contamination of urethral catheters.21–23
The treatment of CRUTI is conditioned by the evolution of the resistances of the most frequent pathogens. Until recently, the quinolones and semisynthetic penicillins were the treatment of choice in the presence of gramnegative bacilli in urine staining. At present, in those cases where CRUTI is accompanied by signs of severe sepsis or septic shock, empirical treatment must consider the presence of extended spectrum betalactamase (ESBL)-producing E. coli (resistant to cephalosporins) or of a non-fermenting gramnegative bacillus (NFGNB), where the combination of a carbapenem and an aminoglycoside active against NFGNB could be the treatment of choice. This combination cannot be maintained for the full treatment period, however, and must be adjusted as soon as possible to the sensitivity profile of the causal pathogen. In cases characterized by the presence of yeasts, the treatment of choice is fluconazole. In contrast, in those situations in which CRUTI is not associated to a systemic inflammatory response, the possibility of not prescribing antimicrobial treatment may be considered.
The mortality rate and duration of stay in the ICU in patients with CRUTI are greater than in patients without this type of infection, though from the information obtained in our study it cannot be deduced that CRUTI increases either mortality or the duration of admission. More complex studies, based on case-control methodology and with patient populations more homogeneous than in our series would be needed in order to draw such conclusions. The published data referred to mortality are contradictory. Recently, a metaanalysis has been published that suggests a statistically significant impact upon mortality and upon the duration of admission to the ICU and hospital stay–though the analysis fails to specify whether use was made of those studies that perform adjustments for the included variables. A probability of in-ICU mortality of 1.94 (95%CI: 1.61–2.34) was identified.24,25
Like all multicenter studies involving voluntary participation, our work is affected by patient selection bias that is offset by the broad participation of ICUs and the volume of patients accumulated. On the other hand, application of the definition of CRUTI in the critical patient (often sedated and/or subjected to analgesia) is difficult, particularly in reference to differentiation with respect to asymptomatic bacteruria; the true rates therefore may have been overestimated. This circumstance is aggravated by the increasing tendency to conduct active multiresistant flora vigilance studies that include urine samples from asymptomatic patients or individuals with other infectious foci.
It can be concluded that CRUTI shows a significant decrease in incidence in Spain, without the adoption of any specific interventions designed to secure such a decrease. The distribution of causal pathogens remains stable, with a predominance of gramnegative bacilli (particularly E. coli). Empirical treatment of the more serious cases must consider coverage of multiresistant pathogens that have become increasingly prevalent–particularly E. coli and P. aeruginosa–while in patients without a systemic inflammatory response the possibility of not prescribing antimicrobial treatment may be considered.
Conflicts of interestThe authors declare that they have no conflicts of interest.
The list of participants in the Study Group on Nosocomial Infection Surveillance ICU between 2005 and 2010 (inclusive) is available in the published reports of ENVIN to which can be accessed from the web: http://hws.vhebron.net/envin-helics/.
Please cite this article as: Álvarez-Lerma F, et al. Infección urinaria relacionada con sonda uretral en pacientes críticos ingresados en UCI. Datos descriptivos del estudio ENVIN-UCI. Med Intensiva. 2013;37:75–82.