Urinary tract infections (UTIs) account for 20–50% of all hospital-acquired infections occurring in the Intensive Care Unit (ICU). In some reports UTI was found to be more frequent than hospital-acquired pneumonia and intravascular device bacteremia, with a greater incidence in developing countries. The risk factors associated with the appearance of UTI include the severity of illness at the time of admission to the ICU, female status, prolonged urinary catheterization or a longer ICU stay and poor urinary catheter management - mainly disconnection of the closed system. The present study offers data on the epidemiology of UTI in the ICU, the identified risk factors, etiology, diagnosis, impact upon morbidity and mortality, and the measures to prevent its appearance.
Dentro de las infecciones intrahospitalarias que ocurren en la Unidad de Cuidados Intensivos (UCI), la infección de vías urinarias (IVU) corresponde a entre el 20 y el 50% del total y, en algunos reportes, es más frecuente que la neumonía nosocomial y bacteriemia asociada a dispositivos intravasculares, con una incidencia que además es mayor en países en vías de desarrollo. Dentro de los factores de riesgo asociados están la severidad de la enfermedad al momento de la admisión, el sexo femenino, una mayor duración del tiempo de cateterización y/o de estancia en UCI y el cuidado del catéter, principalmente el no mantener un sistema de drenaje cerrado, entre otros. En el presente documento se presentan datos sobre la epidemiología de la colonización de la vía urinaria en UCI, los factores de riesgo asociados, la etiología, el diagnóstico, el impacto sobre morbimortalidad y las medidas de prevención.
Intensive Care Units (ICUs) see an important number of in-hospital infections, with a high incidence of multiresistant microorganisms. Among the global infections seen in the ICU, urinary tract infections (UTIs) are among the most common, and are particularly associated with frequent bladder catheter use in critically ill patients. Many studies have investigated the associated risk factors, the impact of such infections upon patient morbidity–mortality and hospital costs, and the measures designed to prevent such infections. However, there is a lack of standardized protocols in a large proportion of ICUs, with little effective knowledge among the hospital personnel of the means available for reducing the appearance of these infections. The present study reviews the evidence on colonization of the urinary tract in the ICU, including asymptomatic bacteruria and UTI, since most of the available literature makes no distinction between the two conditions –this possibly reflecting the existence of a continuum indicating the presence of microorganisms in the urinary tract, with or without patient symptoms.
EpidemiologyApproximately 8–15% of all hospital admissions correspond to admission to Intensive Care; however, ICUs see a comparatively large number of nosocomial infections, mainly associated to the use of invasive medical devices, resulting in associated increases in morbidity, mortality and hospital costs. It has been reported that UTIs represent 20–50% of all these infections,1,2 with a raw incidence of 7–31%.3,4 The recorded incidences in turn are lower in the more developed countries.
Corresponding to the period between 1992 and 1997, the National Nosocomial Infections Surveillance (NNIS) system of the United States compiled data on 112 clinical ICUs in 97 hospitals, with a total of 181,993 patients and 715,930 patients/day of follow-up. The survey registered a total of 14,177 reports of in-hospital infections, of which UTIs were the most frequent (representing 31% of all reported infections), followed by nosocomial pneumonia and primary bacteremia.1
In the year 2008, the International Nosocomial Infection Control Consortium (INICC), a multicenter, international control and vigilance program, published the data on 98 ICUs, including surgical, medical, coronary and neurosurgical units pertaining to public and private hospitals in 18 countries. The report covered the vigilance period between 2002 and 2007, and informed of a total of 1312 UTIs during a total of 202,311 days/catheter over follow-up, with a mean rate of 6.49 infectious episodes per 1000 days/catheter. Considering the global ICUs, the largest presence of UTIs corresponded to clinical and neurosurgical units, with 9.63 and 8.29 infections per 1000 days/catheter, respectively.5 Previous studies had suggested that the nosocomial infection rates differ according to the type of ICU involved, and the findings of the above mentioned vigilance study confirmed this idea. Such differences reflect the existence of differences among the units in relation to epidemiological parameters and in the required control measures.
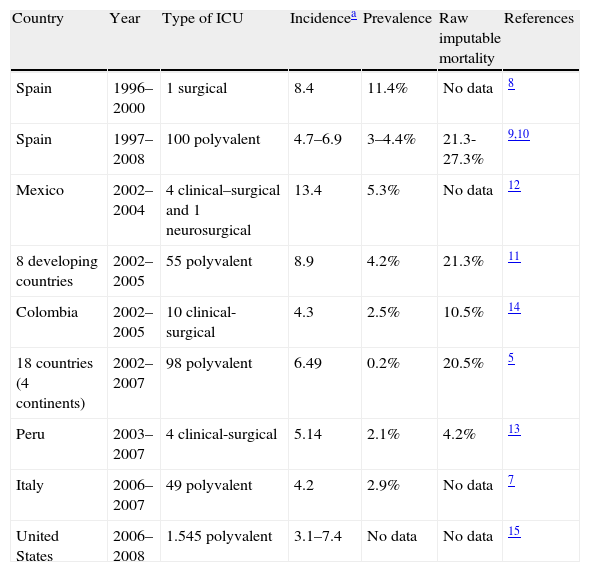
Table 1 provides epidemiological information from different countries and vigilance systems, including cardiovascular Intensive Care,6 Intensive Care in Italy,7 surgical ICUs in Spain,8 and data from the EPINE nosocomial infections network9 and the Spanish National Study of Control of Nosocomial Infection in Intensive Care Units (ENVIN-UCI).10 Information is also presented from developing countries that generally show the incidence of nosocomial urinary infections to be greater than that in industrialized countries.11–14 Data from the North American vigilance systems in turn describe a mean incidence of 3.1 cases per 1000 days of exposure, with much higher rates in burn or neurological units.15
Epidemiology of urinary tract infections associated to bladder catheter use in Intensive Care Units.
| Country | Year | Type of ICU | Incidencea | Prevalence | Raw imputable mortality | References |
| Spain | 1996–2000 | 1 surgical | 8.4 | 11.4% | No data | 8 |
| Spain | 1997–2008 | 100 polyvalent | 4.7–6.9 | 3–4.4% | 21.3-27.3% | 9,10 |
| Mexico | 2002–2004 | 4 clinical–surgical and 1 neurosurgical | 13.4 | 5.3% | No data | 12 |
| 8 developing countries | 2002–2005 | 55 polyvalent | 8.9 | 4.2% | 21.3% | 11 |
| Colombia | 2002–2005 | 10 clinical-surgical | 4.3 | 2.5% | 10.5% | 14 |
| 18 countries (4 continents) | 2002–2007 | 98 polyvalent | 6.49 | 0.2% | 20.5% | 5 |
| Peru | 2003–2007 | 4 clinical-surgical | 5.14 | 2.1% | 4.2% | 13 |
| Italy | 2006–2007 | 49 polyvalent | 4.2 | 2.9% | No data | 7 |
| United States | 2006–2008 | 1.545 polyvalent | 3.1–7.4 | No data | No data | 15 |
Urinary tract infection (UTI) episodes per 1000 days/catheter.
Colonization of the urinary tract is the step prior to infection. Asymptomatic bacteruria is defined as the isolation of a specific amount of bacteria from an adequately collected urine sample, obtained from a patient without clinical signs or symptoms of urinary infection. The amount depends on the sample collection method used, and the corresponding cutoff points can be found elsewhere.16 In the general population, the prevalence of asymptomatic bacteruria ranges from 1 to 100%, depending on the characteristics of the study population, and it is greater in women than in men, increases with age, and mainly affects pregnant women (2–9.5%), diabetic subjects (0.7–27%), patients with spinal cord injuries (23–89%), and patients with a permanent bladder catheter (100%).16 However, there are no clear data on the incidence of asymptomatic bacteruria in hospitalized patients. In the United States NNIS report, 62% of the patients presented symptoms, defined as fever or lower urinary tract symptoms –this implying a 38% prevalence of patients with probable asymptomatic bacteruria.1 In one German hospital, 77% of the patients with colonization/infection of the urinary tract presented no symptoms.2
Risk factorsIn order to evaluate the possible risk factors associated to colonization of the urinary tract in ICUs, mention must be made of the three proposed physiopathological mechanisms involved, since such infections can develop in three different ways: (a) colonization through the catheter lumen when the catheter is removed from the collector bag (a situation that should not happen); (b) colonization of the urinary meatus by gastrointestinal tract bacteria, which ascend along the external catheter wall (this representing 66% of all cases)17; and (c) colonization from a remote location. This mainly occurs in relation to bloodstream infections produced by Staphylococcus aureus and candidemias.18
A number of studies have proposed different risk factors associated to colonization of the urinary tract in the ICU, and which could contribute to define possible interventions designed to lessen their impact. Among the risk factors that have been related to colonization of the urinary tract in the ICU, special mention must be made of bladder catheters.3,19 In the NNIS report and in a study published by Van Der Kooi et al. in Holland, a full 95% of all urinary tract infections were associated to bladder catheters,1,4 thus evidencing the importance of these devices in relation to urinary infection. There is an average 2–6% colonization rate for every day with the bladder catheter in place. Consequently, it can be estimated that 100% of the patients are colonized after 20 days of catheterization. If we moreover consider that the great majority of patients admitted to the ICU receive a bladder catheter, and that approximately 16–28% of these subjects develop UTIs,20,21 we can understand the high prevalence of urinary infections in the ICU. The importance of keeping the urinary drainage system closed (a protective factor identified in the 1960s), is seen in the data generated by the Spanish vigilance system. In effect, during the 1990s, with an increase (from 56% to 69%) in the use of closed urinary drainage systems in the ICU, the prevalence of UTIs in this scenario dropped by almost 50%.22
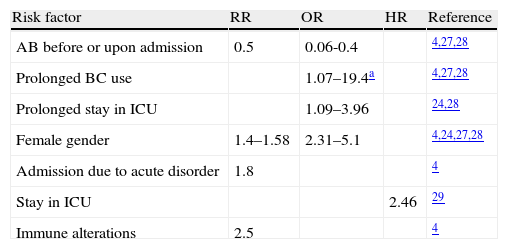
Many studies have examined other risk factors, including severity of the disease upon admission, the female gender, and an increased duration of catheterization and/or stay in the ICU.23,24 Some authors have pointed to previous or concurrent antibiotic use, older patient age, and catheter care as important risk factors.25 In the study published by Van Der Kooi et al., including the data from 23 ICUs, a strong correlation to the duration of bladder catheterization was found, with an odds ratio (OR) of 1.6 and 3.3 for 5–9 days and over 10 days, respectively. In turn, the female gender, immune alterations, acute ICU admission, and the non-utilization of systemic antibiotics at the time of admission have been cited as independent risk factors for UTI in the ICU. However, it is important to mention that although antibiotic use at the time of admission was found to protect against UTI, it was identified as a mortality risk factor in the multivariate analysis.4 Another study conducted in Marseille (France) in a polyvalent ICU identified the following parameters as independent risk factors for the development of UTI in the ICU: the duration of stay in the ICU, the duration of bladder catheterization, the female gender, and the severity of the disease as measured with the SAPS II score. Here again, antibiotic use was identified as a protective factor.26 These findings are very similar to those published for a French clinical ICU, where the risk factors for bacteruria associated to bladder catheter (without discriminating between patients with and without UTI) were the duration of bladder catheterization (OR=19.4 for ≥11 days) and the female gender. Here again, prior antibiotic use was identified as a protective factor (OR=0.06).27,28 In another North American study, conducted in a trauma ICU, the multivariate analysis identified age (>60 years), prolonged ICU and hospital admission, disruption of the closed drainage system, and the duration of bladder catheterization as risk factors associated to urological sepsis in critical patients with a bladder catheter.20 In contrast, no studies have reported diabetes mellitus, urological structural anomalies or alterations in urinary flow to be correlated to an increased incidence of UTI3 (Table 2).
Identified risk factors for the development of catheter-related urinary tract infections in Intensive Care Units.
| Risk factor | RR | OR | HR | Reference |
| AB before or upon admission | 0.5 | 0.06-0.4 | 4,27,28 | |
| Prolonged BC use | 1.07–19.4a | 4,27,28 | ||
| Prolonged stay in ICU | 1.09–3.96 | 24,28 | ||
| Female gender | 1.4–1.58 | 2.31–5.1 | 4,24,27,28 | |
| Admission due to acute disorder | 1.8 | 4 | ||
| Stay in ICU | 2.46 | 29 | ||
| Immune alterations | 2.5 | 4 |
AB: antibiotic; HR: hazard ratio; OR: odds ratio; RR: relative risk; BC: bladder catheter.
11 days or more.
A study carried out in Israel found the UTI rate to be greater in the ICU than in the hospitalization wards, among patients meeting criteria for admission to the ICU (3.28 versus 1.27 per 100 days and risk patient, respectively). The importance of this study is that it pointed to admission to the ICU as an independent risk factor for the development of UTI, even after the multivariate analysis to adjust for confounding factors. These observations are probably due to the differences in the duration of bladder catheterization and catheter care, which were not included in the analysis.29
In developing countries, the incidence of nosocomial infections associated to medical devices is 3- to 5-fold higher than that in developed countries. The factors that can explain these differences are the lack of in-hospital infection control programs and limitations in resources –including the availability of glycerinated alcohol– the few existing accredited hospital institutions, the low nurse/patient ratio, the high proportion of nursing personnel with limited experience, and the use of obsolete technology.5
EtiologyMost urinary infections, whether community acquired or nosocomial, are of a monomicrobial nature, while 5–12% are caused by multiple bacterial species. The distribution of the different microorganisms and their resistance profiles depend on the local epidemiological circumstances. According to the international literature, the main microorganisms isolated are Escherichia coli (E. coli), Pseudomonas aeruginosa and Enterococcus spp.,23,26 with a prevalence of Candida spp. that can reach one-third of all the urinary infections acquired in the ICU.3,30 In the NNIS report, the most frequently isolated microorganism was Candida albicans (C. albicans) (21%), followed by E. coli and Enterococcus spp., with equal frequencies (14% in both cases).1 In the ENVIN-UCI report, the predominant microorganisms were gramnegative bacilli (56.9%), fundamentally E. coli and Pseudomonas aeruginosa, followed by fungal species (23.6%), mainly in the form of C. albicans, which represented 13.2% of the cases.9 In turn, the INICC report found the two most frequently isolated groups of microorganisms to be the enterobacteria and C. albicans (42% and 30%, respectively).11
DiagnosisWe have found very few specific studies on the diagnostic performance or yield of urine tests, reactive strips, urine cultures and urine Gram staining in critically ill patients. The existing information corresponds to outpatients or patients seen in the Emergency Department; in the present review we have therefore extrapolated these data.
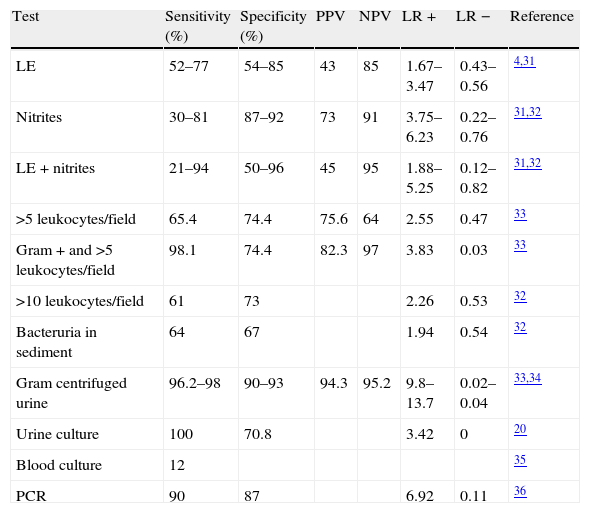
Evaluations have been made of the performance of reactive strips,31 as well as of several individual tests such as the nitrite and pyuria techniques (positivity being defined on the basis of over 10 leukocytes per microscopic field).32 Although the performance of these techniques alone or in combination is highly variable, in general the best option is gram staining of centrifuged urine (Table 3).33,34
Diagnostic yield of the test use to identify urinary tract infection.
| Test | Sensitivity (%) | Specificity (%) | PPV | NPV | LR + | LR − | Reference |
| LE | 52–77 | 54–85 | 43 | 85 | 1.67–3.47 | 0.43–0.56 | 4,31 |
| Nitrites | 30–81 | 87–92 | 73 | 91 | 3.75–6.23 | 0.22–0.76 | 31,32 |
| LE+nitrites | 21–94 | 50–96 | 45 | 95 | 1.88–5.25 | 0.12–0.82 | 31,32 |
| >5 leukocytes/field | 65.4 | 74.4 | 75.6 | 64 | 2.55 | 0.47 | 33 |
| Gram+and >5 leukocytes/field | 98.1 | 74.4 | 82.3 | 97 | 3.83 | 0.03 | 33 |
| >10 leukocytes/field | 61 | 73 | 2.26 | 0.53 | 32 | ||
| Bacteruria in sediment | 64 | 67 | 1.94 | 0.54 | 32 | ||
| Gram centrifuged urine | 96.2–98 | 90–93 | 94.3 | 95.2 | 9.8–13.7 | 0.02–0.04 | 33,34 |
| Urine culture | 100 | 70.8 | 3.42 | 0 | 20 | ||
| Blood culture | 12 | 35 | |||||
| PCR | 90 | 87 | 6.92 | 0.11 | 36 |
LE: leukocyte elastase; LR: likelihood ratio; PCR: polymerase chain reaction; NPV: negative predictive value; PPV: positive predictive value.
Urine culture has been regarded as the gold standard for diagnosing UTI, with a cutoff number of colony-forming units (CFU) that depends on the presence or absence of a bladder catheter: in patients without a bladder catheter, the cutoff value is 105CFU with the isolation of fewer than two microorganisms, while in patients with a bladder catheter in which sampling is performed using an aseptic technique, the cutoff value is 103CFU.16 A study involving 126 patients meeting criteria of sepsis in an ICU in the United States evaluated the performance of urine testing in diagnosing urological sepsis. The sensitivity of urine testing combined with urine culture was found to be 100%, with a specificity of 24.1% in reference to urine testing and of 70.8% in reference to urine culture.20 This study identified a 61% frequency of asymptomatic bacteruria, which lowers the positive predictive value of culture in the absence of appropriate clinical manifestations and the exclusion of other possible diseases.
A study conducted in Nashville (the United States) in turn examined the usefulness of blood cultures in patients with a clinical diagnosis of pyelonephritis and positive urine culture. Blood culture proved positive in 18% of the total patients, and 32% of the isolates corresponded to coagulase-negative Staphylococcus strains, which were interpreted as representing contamination –the true diagnostic yield therefore being 12%.35
Taking into account that antibiotic treatment is generally started on an empirical basis, and that identification of the causal microorganism is very important in order to guide antibiotic treatment, a study was carried out in Switzerland to evaluate the usefulness of the polymerase chain reaction (PCR) technique applied to 301 samples from outpatients and patients admitted to the ICU, comparing the data obtained with the urine culture findings. The recorded sensitivity was 90%, with a specificity of 87%, and a correlation of 95.8% and 57.9% in the case of mono- and polymicrobial infections, respectively.36 However, the availability of PCR is still somewhat limited, and its associated costs reduce the applicability of the technique. In any case, PCR testing must be taken into account in future studies, since it offers the advantage of faster results compared with urine culture, with good correlation in monomicrobial infections.
ImpactThe impact of nosocomial urinary tract infections is reflected by the generated costs, hospital stay and mortality. Unfortunately there is no specific information on the cost of each episode of urinary tract infection in the ICU. Data on nosocomial urinary infection in the hospitalized patient (without distinguishing between admission to the ICU or to other hospital areas) have revealed an increase in the costs associated with diagnosis and drug treatment.37,38
In the study carried out by Van Der Kooi in Holland, a mean hospital stay of 6 days was recorded in patients with a bladder catheter and no UTI versus 18.5 days in those with UTI.4 An Egyptian study in four ICUs in turn reported a statistically significant difference between the patients with UTI and those without UTI. The average difference was found to be four days.39
Regarding mortality, the existing articles yield contradictory results. A recent metaanalysis has been published on the studies found in the scientific literature.40 Although most of the evidence points to a statistically significant impact upon mortality, ICU stay and hospital stay, these findings were not maintained on considering the studies that performed adjustments of the included variables. The likelihood ratio identified for mortality in the ICU was 1.94 (95%CI: 1.61–2.34). Table 1 also offers information on the mortality rates reported by some studies.
TreatmentTreatment measures are only indicated in patients with urinary infection, i.e., with the presence of symptoms, together with microbiological documentation of bacteruria.16 The treatment of asymptomatic bacteruria has not been shown to offer major clinical benefits in colonized patients, and in fact could contribute to an increase in bacterial resistances in hospital centers. In any case, treatment is to be adjusted to the local epidemiological conditions.
PreventionThe importance of obtaining data on the risk factors for colonization of the urinary tract and for urinary tract infections, and their impact upon the costs, hospital stay and mortality, in turn underscores the importance of prevention. In this context, many studies have described interventions designed to reduce the incidence of bacteruria or its progression to UTI and sepsis.
The main recommendation is to maintain a closed sterile system. However, the use of simple or complex closed systems yielded no significant differences in a non-randomized comparative study published by Leone et al.41 This finding was posteriorly confirmed by the same group in the context of a randomized study in which the recorded incidence of bacteruria was 8.0% versus 8.6% in the patients with a two-chamber drainage system and a complex drainage system, respectively. The best drainage method therefore remains the simple closed system.42
The usefulness of bladder catheters impregnated with hydrogels and silver salts is not clear. A randomized study found no benefits in terms of the urinary tract infection rate,43 and a study carried out in 5 centers with masking during an intervention period likewise recorded no decrease in the UTI rate in the multivariate analysis– the rate being 8.1 cases per 1000 days/catheter in the baseline period and 4.9 cases per 1000 days/catheter during the intervention period (p=ns).44 A randomized study of silver-impregnated catheters was published in the year 2000. The cost-effectiveness analysis revealed a rate of 1.1UTIs per 100 patients in the treatment group versus 1.36 in the control group, with a RR=0.81, but with a nonsignificant confidence interval (95%CI: 0.65–1.01). However, there was a difference in the cumulative incidence of UTI, with 2.66 cases per 1000 days/catheter in the treatment group versus 3.35 in the control group, with RR=0.79 (95%CI: 0.63–0.99; p=0.04), and with a decrease in costs associated to nosocomial UTI of between 14,456 and 573,293USD a year.45 A systematic review has also been published, examining 12 randomized or pseudorandomized studies analyzing silicone catheters coated with nitrofurazone and silver-coated latex catheters, in hospitalized patients. One of the main problems, in addition to the quality of the included studies, was the fact that one publication corresponded to patients in the ICU or neurosurgical unit, while the rest failed to specify whether critically ill patients were included. The conclusion of the review was that bladder catheters with antimicrobial agents may prevent or delay the appearance of catheter-related bacteruria in selected hospitalized patients, though the magnitude of the effect varies according to the type of catheter involved, the year of publication and other variables. These factors may have led to over-estimations, and moreover the effects upon morbidity and bacteremia were not evaluated.46 The use of hydrophilic bladder catheters likewise has not yielded successful results. In a small-randomized study of spinal injury patients, the use of hydrophilic bladder catheters was not associated with a decrease in the frequency of UTI.47 In 2007 a randomized, double-blind and controlled study evaluated the usefulness of bladder catheters impregnated with nitrofurazone in reducing bacteruria and funguria. A reduction was observed in the incidence of bacteruria and funguria per 1000 days/catheter with the impregnated catheters versus the silicone catheters (13.8 per 1000 days/catheter versus 38.6 per 1000 days/catheter), with an OR=0.31 (95%CI: 0.14–0.70). However, on evaluating hospital stay, stay in the ICU, and mortality after 30 days, no significant differences were observed.48 In the light of the existing information, it is not possible to recommend the generalized use of these bladder catheters.
Daily disinfection of the urethral meatus has been evaluated, though this likewise has failed to reduce the incidence of UTIs. In 1992 a study was published involving 696 patients admitted to hospital (not to the ICU) and randomized to receive 2.5ml of 1% silver sulfadiazine, twice a day applied to the urethral meatus. The incidence of bacteruria was 11.4% and 13.2% in the intervention and non-intervention groups, respectively, without statistically significant differences (p=0.56; OR=0.85; 95%CI: 0.53–1.37).49 A pilot study carried out in Turkey assigned 130 patients to 5 different groups: 1) once daily application of 9% iodine–povidone, 2) twice daily application of 9% iodine–povidone, 3) once daily application of 4% chlorhexidine, 4) twice daily application of 4% chlorhexidine, and 5) control group. There were no significant differences in the incidence of UTI, or in the microorganisms isolated.50 Therefore, on the basis of the existing evidence, we are likewise unable to recommend routine disinfection of the urethral meatus with any concrete antiseptic agent.
Bladder irrigation with neomycin–polymyxin or iodine–povidone in hospitalized patients was not found to reduce the UTI rate, and in fact neomycin–polymyxin increased the incidence of resistant microorganisms in the urinary tract.18 This approach is therefore also not advisable. The use of systemic antibiotics was evaluated in two important studies. The first was a randomized trial carried out by Leone et al., in which 60 patients admitted to a clinical–surgical ICU with asymptomatic bacteruria were assigned to three days of antibiotic treatment plus bladder catheter replacement, or to neither of the two interventions. The antibiotics were prescribed according to the established susceptibility profile. The two interventions were not seen to reduce the urinary sepsis rate, the stay in the ICU, or patient mortality. It was therefore concluded that the administration of systemic antibiotics in patients with asymptomatic bacteruria in the ICU is not advisable,51 particularly when moreover also considering the potential effects in terms of bacterial resistance that have been reported in other studies.52 The second study examined the usefulness of antibiotic prophylaxis before removal of the bladder catheter in reducing the UTI rate in surgical patients. A total of 239 patients were randomized to trimethoprim/sulfamethoxazole for three days before removing the bladder catheter, or to no treatment. The UTI rate was found to be 4.9% in the intervention group versus 21.6% in the control group, with an absolute risk reduction of 16.7% and a number needed to treat (NNT) of 6.53 Based on these findings, it would be advisable to administer prophylactic antibiotic treatment before bladder catheter removal, with no antibiotic treatment for the detected bacteruria episodes. However, studies involving larger patient series and longer durations of follow-up are needed, together with replication of the mentioned trial in other scenarios involving different resistance patterns, in order to assess the effects of prophylaxis upon hospital stay, mortality and bacterial resistance. Despite these findings, a study on the perception of UTI in ICUs carried out in Canada, based on a survey of 90 physicians with training in Intensive Care, found that although bacteruria is perceived as being a low morbidity–mortality problem by 63% of those surveyed, 19% of them would administer antibiotics to an asymptomatic patient, and a full 98% used no protocol for the management of bacteruria/UTI in the ICU.54 This shows that despite the existing knowledge on the absence of benefit of treating asymptomatic bacteruria in the ICU, management protocols need to be standardized.
On reviewing the available evidence on measures for controlling bladder catheter-related UTI, it can be concluded that the only measures shown to be of use are maintaining the drainage system closed, and the expert recommendations summarized in two clinical practice guides: that of the Healthcare Infection Control Practices Advisory Committee/Centers for Disease Control (HICPAC/CDC) for the prevention of UTI associated to bladder catheter, published in 2010, and the guidelines of the Infectious Diseases Society of America (IDSA) for the diagnosis, prevention and treatment of UTI associated to catheters in adults, likewise published in 2010. The recommendations of the two mentioned guides are very similar, and in general lines consist of the following: 1) use of a bladder catheter only when the indications are clear, removing it when no longer needed, using the minimum required caliber, and considering alternatives to catheterization in selected patients; 2) washing of the hands before and after catheter insertion and manipulation; 3) handling of the drainage systems by personnel trained in aseptic techniques; 4) insertion of the catheter using an aseptic technique; 5) adequate catheter fixation to avoid movement and urethral traction; 6) keeping the drainage system closed, and replacing the catheter and collector system by means of an aseptic technique if system closure is disrupted (with consideration of the use of sealed, pre-connected systems); 7) avoidance of kinking of the collector tube, regularly voiding the collector bag in a separate container, avoiding contact between them, irrigating or replacing obstructed or malfunctioning catheters, and keeping the collector bag below bladder level at all times, without resting it on the floor; 8) use of standard precautions in any manipulation of the catheter or collector system; 9) replacement of the catheters or collector bags not on a routine basis but when there is infection, obstruction or loss of the closed system condition; 10) avoidance of routine systemic antibiotic use to prevent UTI and of antiseptics to clean the urethral meatus, avoiding also bladder irrigation with antiseptics or antiseptic instillation in the collector bag; 11) avoidance of bladder irrigation unless obstruction is expected, as in the postoperative period of prostatectomy or of bladder surgery, where continuous closed circuit irrigation is indicated; 12) catheter replacement in the event of obstruction; 13) avoidance of catheter clamping before removal; 14) consideration of the use of antibiotic-impregnated catheters if the previous strategies fail to reduce the UTI rate; 15) use of the sampling port, following disinfection of the latter, without disconnecting the system (if large urine samples are required, these should be collected aseptically from the collector bag); 16) introduction of quality programs to reinforce correct usage and pertinent urinary catheter withdrawal, including management guides and algorithms, and training programs; 17) consideration of the use of patient follow-up sheets including the indications of insertion, the date, the person placing the catheter, and the date of withdrawal; 18) guarantee of the availability of supplies to maintain the aseptic condition of the techniques; 19) generation of vigilance programs for evaluating the incidence of UTI and the bladder catheter utilization coefficient, among other variables; and 20) avoidance of routine asymptomatic bacteruria screening practices.55,56
The usefulness of hand washing and keeping the drainage system free of obstruction was evaluated in Argentina by Rosenthal et al., who recorded a decrease from 21.3UTI episodes per 1000 days/catheter to 12.39 episodes per 1000 days/catheter in the post-intervention period, with RR=0.58 (95%CI: 0.39–0.86)25– this once again showing that in our setting, vigilance programs and nursing personnel management guide feedback measures influence the incidence of UTI.
In conclusion, UTIs and asymptomatic bacteruria are highly prevalent in the hospital setting, and particularly in the ICU. The incidence is higher in developing countries, due to a number of factors that range from the lack of vigilance programs to a limited availability of different technologies. In addition, such infections have a strong impact upon morbidity, and possibly also on mortality, causing a prolongation of hospital stay and a possible increase in imputable mortality. They also exert an important influence in terms of hospital costs. The risk factors underlying these infections have been found to be very similar in the different studies published to date, and include particularly the use of a bladder catheter, the duration of catheterization, the length of stay in the ICU, the female gender, the severity of the background disease, and catheter care. The isolated microorganisms have been the same in the different series, with a high prevalence of enterobacteria, followed by Pseudomonas spp., Enterococcus spp. and Candida spp. The measures for preventing these infectious processes include mainly a correct indication for bladder catheter use, timely removal of the catheter, and the optimization of catheter care. In this context, emphasis should be placed on the need to keep a closed drainage system, with the creation of protocols, management guides and training programs for the nursing personnel. Further studies in turn are needed to establish the true usefulness of antibiotic-impregnated catheters, system irrigation with antibiotics, or periodic disinfection of the urethral meatus –these being measures which to date have not been found to be effective in reducing the presence of bacteruria or UTI.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: López MJ, Cortés JA. Colonización e infección de la vía urinaria en el paciente críticamente enfermo. Med Intensiva. 2012;36:143–51.