To describe the apparent diffusion coefficient (ADC) in a series of severe traumatic brain injuries, their clinical and outcome features, and possible implications.
DesignA descriptive, observational case-series study was carried out.
Patients and interventionsPatients with severe traumatic brain injuries (TBIs) admitted to the ICU were subjected to MRI study using a 1.5T scanner. Diffusion-weighted images (DWMR) were obtained using the following echo-planar pulse sequence: TR 10,000ms, TE 126.9ms, with b values 1000s/mm2 in the three spatial dimensions. Combining the three sets of images, an isotropic image conforming a map of the mean ADCs was obtained.
ResultsDWMR was performed in 23 patients with severe TBI admitted to the ICU between 2001 and 2004. In the MR images we selected 26 regions of interest (ROIs) where ADC was recorded. We observed a clear increase in diffusion in non-treated space-occupying lesions versus other types of injuries and the normal values. A poorer outcome was recorded in patients with lower ADC values.
ConclusionsMean ADC in the lesions was greater than the normal values and greater in contusions than in other types of injuries, as an expression of extracellular edema. ADCs were decreased in patients with a poor outcome, suggesting an association between ischemia and the patient prognosis.
Describir el coeficiente de difusión aparente (CDA) medido mediante resonancia magnética potenciada en difusión (RMD) en una serie de pacientes con traumatismo craneoencefálico (TCE) grave, sus aspectos clínicos y pronósticos y sus posibles implicaciones.
DiseñoEstudio observacional, descriptivo de serie de casos.
Pacientes e intervencionesPacientes con TCE grave, ingresados en UCI que no presentan contraindicaciones para traslado y realización de resonancia magnética (RM). Se realizó RM usando un scanner de 1.5Tesla. Las imágenes potenciadas en difusión se obtuvieron usando una secuencia de pulso eco-planar con las siguientes características: TR 10.000ms, TE 126,9ms, valores b 1.000s/mm2 en las tres direcciones del espacio. Combinando los tres sets de imágenes se obtuvo una imagen isotrópica que constituyó el mapa de los CDA medios.
ResultadosRMD se realizó en 23 pacientes con TCE grave ingresados en UCI entre 2001 y 2004. Se seleccionaron para su análisis 26 regiones de interés y se recogió el CDA en cada una de ellas. Se observó un incremento en la difusión en las lesiones ocupantes de espacio no evacuadas respecto a otros tipos de lesión y a valores normales. El pronóstico, según la escala de resultados de Glasgow, fue peor en los pacientes con valores de CDA más bajos.
ConclusionesLos CDA medios fueron mayores que los valores normales y mayores en las contusiones que en otros tipos de lesión, como expresión de edema extracelular. Los CDA estaban disminuidos en pacientes con mal pronóstico sugiriendo una asociación entre isquemia y pronóstico.
The mortality statistics of severe traumatic brain injury (TBI) have changed very little in the last 20 years. The principal mechanism leading to death is uncontrolled intracranial hypertension and the ischemic brain damage it produces.1 The cause of such intracranial hypertension is the increase in brain volume triggered by different mechanisms, including vasogenic or extracellular brain edema secondary to rupture of the blood–brain barrier (BBB) and cytotoxic or intracellular edema, associated to ischemia. Intracellular edema is considered to be the main contributor to brain swelling after closed trauma.2–4
Diffusion-weighted magnetic resonance imaging (DWMR) produces a signal with an intensity proportional to the diffusion of water molecules in the tissues. The signal depends on the diffusion capacity of the molecules, on the interactions among them, and on the obstacles facing molecular movement. The intensity of the signal can be quantified by the apparent diffusion coefficient (ADC), which is calculated by means of the following formula: ADC=(1/b)log(S1/S0), where “b” is a factor that depends on the strength and duration of the pulses applied to increase diffusion, “S1” is the intensity of the diffusion-weighted signal, and “S0” is the intensity of the non-diffusion-weighted signal. If this formula is applied to each voxel (three-dimensional graphic element), the end result is a map or image in which the intensity of the signal is proportional to the ADC of each region or tissue examined.5–8
Conventional magnetic resonance imaging (MRI) is unable to distinguish between types of edema based on the intensity of the signal; both types of edema, cytotoxic and vasogenic, produce a hyperintense signal in T2-weighted sequences. In contrast, DWMR differentiates cytotoxic edema, which is characterized by an increase in intracellular fluid uptake, resulting in low ADC values. The ADC is reduced as a consequence of restricted water molecule diffusion produced by the membranes and organelles within the cell, and by the increase in viscosity.
In contrast, in vasogenic edema, the fluid displaces from the vessels to the extracellular compartment, which therefore increases in size and allows the molecules to move more freely. This produces different effects in the magnetic resonance image: either high signal intensity in the ADC and DWMR maps or, less frequently, high signal intensity in the ADC map and low signal intensity in the DWMR image (facilitated diffusion). These changes in signal intensity are generally located around a focal parenchymal lesion, for example, a hematoma, since the blood clot exerts an osmotic effect upon the surrounding brain tissues.4,9
To date, the main application of DWMR has been the detection of acute cerebral infarction. In this context, it is able to detect stroke earlier than any other technique, thanks to its high sensitivity, and DWMR is moreover able to predict the infarct size and assess the patient prognosis.10–13
DWMR has been used in patients with TBI to evaluate diffuse axonal injury (DAI), in view of its greater sensitivity compared with other conventional techniques (T2 and FLAIR sequencing) in the acute phase after trauma.14,15 In addition, the volume of the lesion detected with this technique is correlated to clinical outcome.
Since DWMR is able to distinguish between extracellular and intracellular edema, it could be used to evaluate the contribution of both types of edema to the damage observed in patients with severe TBI.16 Furthermore, the early detection of secondary ischemic lesions in severe TBI would be of great importance, given their impact upon morbidity-mortality.
The present study analyzes the ADC values in a series of patients with focal trauma and their correlation to initial clinical severity and ultimate patient prognosis or outcome.
MethodsDesignA descriptive, observational case series is presented.
Patients and clinical dataThe study involved patients with severe TBI, defined by a Glasgow coma score of fewer than 9 without sedation in the first 24h after injury,17 admitted to the Intensive Care Unit (ICU) of Canarias University Hospital (Spain), and with no contraindications to transfer or MRI study.
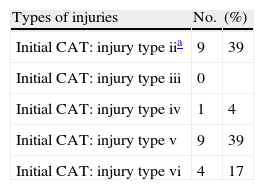
The traumatisms were categorized according to the initial computed tomography (CT) scan, based on the Marshall classification18 (Table 1).
All patients were subjected to early surgical evacuation of space-occupying lesions, with hemodynamic, respiratory and intracranial pressure monitoring. Intracranial hypertension was managed using osmotherapy, optimized hyperventilation, and occasionally barbiturates. The outcome data were evaluated based on the Glasgow Outcome Score (GOS) at discharge from the ICU and after 6 months.19
Imaging techniques and analysisThe MRI studies were made using a 1.5Tesla system (General Electric). The imaging protocol comprised the following sequences: a T1-weighted sagittal sequence, a T1-weighted axial sequence, a T2-weighted axial sequence, and a fluid attenuated inversion recovery (FLAIR) sequence. The diffusion-weighted images were obtained using an echo-planar pulse sequence with the following characteristics: TR 10,000ms, TE 126.9ms, b values 1000s/mm2 in the three spatial dimensions. By combining the three sets of images we obtained an isotropic image conforming a map of the mean ADC values. Image processing for calculation of ADC was made using Functool 2000® software.
In order to analyze the image, we established a measurement zone in each lesion, selecting the section where the diffusion-weighted image presented a greater signal intensity, and within this image we selected a bidimensional region of interest (ROI) where ADC was measured. The core of the hemorrhagic lesions was excluded from the calculation. In the diffuse lesions we measured ADC in one or both hemispheres.
The normal reference values for the mean ADC were those obtained by Helenius et al.,20 who measured the values of 18 neuroanatomical regions in 18 healthy volunteers. The values are the following: (890±40)×10−6mm2/s in the cortical gray matter (range 780–1090×10−6mm2/s), (700±30)×10−6mm2/s in the white matter (range 620–790×10−6mm2/s), (750±30)×10−6mm2/s in the basal ganglia (range 640–830×10−6mm2/s), and (730±30)×10−6mm2/s in the thalamus (range 670–820×10−6mm2/s).
Statistical analysisContinuous variables are reported as the mean and standard deviation, while discrete variables are reported as absolute and relative frequencies.
ResultsWe recorded 71 patients with severe TBI admitted to the ICU between November 2001 and October 2004. DWMR was performed in 23 of them.
Tables 1 and 2 show the epidemiological and clinical data.
Types of injuries according to the Marshall classification.
| Types of injuries | No. | (%) |
| Initial CAT: injury type iia | 9 | 39 |
| Initial CAT: injury type iii | 0 | |
| Initial CAT: injury type iv | 1 | 4 |
| Initial CAT: injury type v | 9 | 39 |
| Initial CAT: injury type vi | 4 | 17 |
| Category | Definition |
| Type i diffuse lesion (no visible alterations) | No intracranial alterations visible in CAT |
| Type ii diffuse lesion | Permeable basal cisterns. Midline deviation is <5mm. No high-density lesions or mixed-density lesions larger than 25ml. Possible bone fragments or foreign bodies |
| Type iii diffuse lesion (swelling) | Compressed or absent cisterns. Midline deviation is <5mm. No high-density lesions or mixed-density lesions larger than 25ml |
| Type iv diffuse lesion | Midline deviation is >5mm. No high-density lesions or mixed-density lesions larger than 25ml |
| Evacuated mass-type lesion | Any surgically evacuated lesion |
| Non-evacuated mass-type lesion | High-density lesions or mixed-density lesions larger than 25ml, not surgically evacuated |
Regarding the types of injuries, surgically evacuated space-occupying lesions were the most frequent presentation, followed by type II lesions. There were no type III diffuse lesions in our series. Seven lesions were defined as diffuse axonal injury (DAI) on the basis of their magnetic resonance location in the subcortical white matter, corpus callosum or brainstem, together with persistent coma (Glasgow coma score<9).
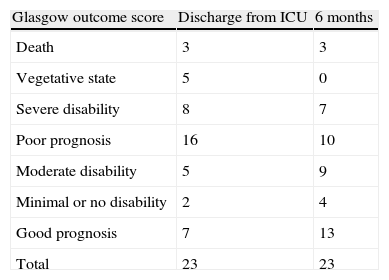
Regarding outcome as determined by the GOS, 70% of the patients had died or were in a vegetative state or seriously disabled (poor prognosis) at discharge from the ICU, versus 43% after 6 months. These results are reported in Table 3.
Attempts were made to perform MRI as soon as possible after admission, though in only four patients was the exploration made within the first 24h, while 12 patients (14 lesions) were subjected to MRI evaluation within the first 48h. In the rest of the cases the timing of the study was highly varied due to different reasons–fundamentally patient instability and the distance to the MRI unit, which according to the physician in charge questioned the usefulness of the study. In any case, we finally decided to analyze all the studies, even if performed over a broad interval of time. Globally, MRI was performed at an average of 101.39±111.80h (range 10–360) after trauma.
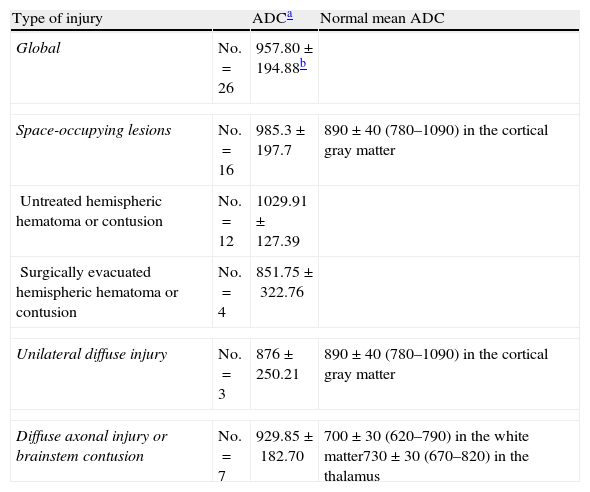
We selected 26 ROIs for analysis in the images of the 23 patients, and documented the ADC in each of them. Table 4 shows the mean values globally and according to the type of lesion. An increase in diffusion in the non-evacuated space-occupying lesions was observed when compared with the rest of the types of injuries and with the normal values.
Apparent diffusion coefficient, global and according to type of injury.
| Type of injury | ADCa | Normal mean ADC | |
| Global | No.=26 | 957.80±194.88b | |
| Space-occupying lesions | No.=16 | 985.3±197.7 | 890±40 (780–1090) in the cortical gray matter |
| Untreated hemispheric hematoma or contusion | No.=12 | 1029.91±127.39 | |
| Surgically evacuated hemispheric hematoma or contusion | No.=4 | 851.75±322.76 | |
| Unilateral diffuse injury | No.=3 | 876±250.21 | 890±40 (780–1090) in the cortical gray matter |
| Diffuse axonal injury or brainstem contusion | No.=7 | 929.85±182.70 | 700±30 (620–790) in the white matter730±30 (670–820) in the thalamus |
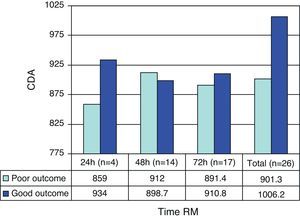
The ADC values showed a progressive increase with the time from trauma–ADC in the first 48h being lower than the values recorded later in time (898.7±205.3/1026.6±164).
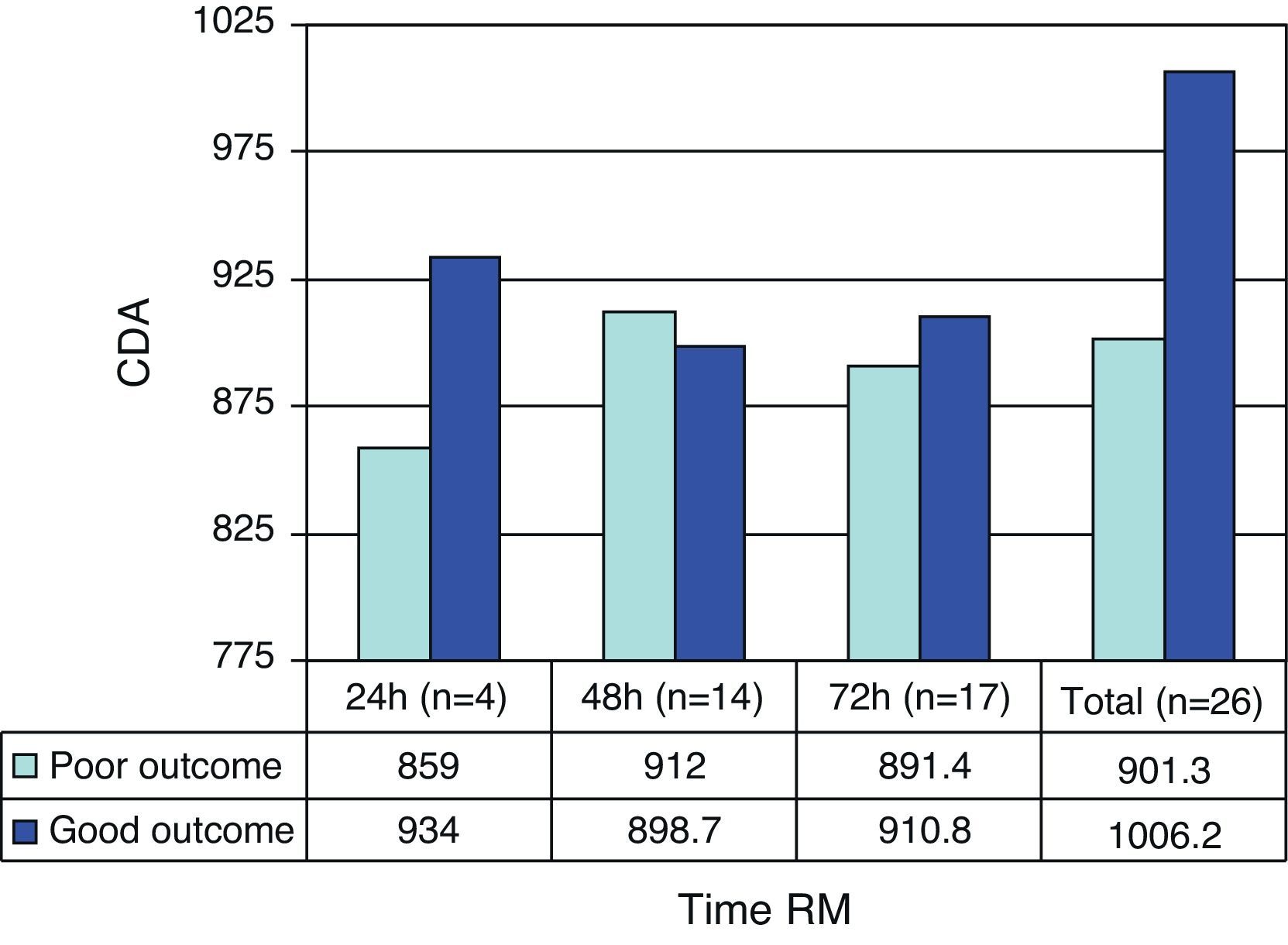
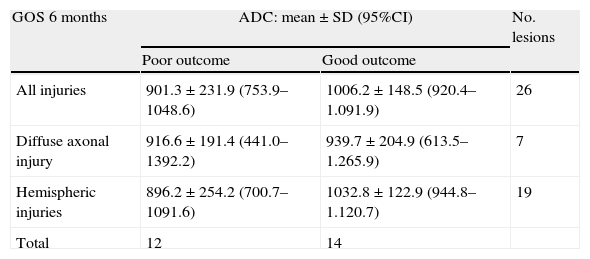
The prognosis (outcome) was considered poor if the patient had died after 6 months or was in a vegetative state or seriously disabled, and good in the case of patients with moderate or slight disability, or no disability. As can be seen in Table 5, the patients with a poor prognosis had lower ADC values than those with a good prognosis. Fig. 1 shows the outcomes according to the ADC findings at the three measurement timepoints. It should be noted that both in the global results and on considering only the measurements made in the first 24h and those made in the first 72h, the ADC values were always lower in the patients with a poor prognosis–though the sample size was too small to allow the drawing of firm conclusions.
Mean ADC value in relation to patient outcome.
| GOS 6 months | ADC: mean±SD (95%CI) | No. lesions | |
| Poor outcome | Good outcome | ||
| All injuries | 901.3±231.9 (753.9–1048.6) | 1006.2±148.5 (920.4–1.091.9) | 26 |
| Diffuse axonal injury | 916.6±191.4 (441.0–1392.2) | 939.7±204.9 (613.5–1.265.9) | 7 |
| Hemispheric injuries | 896.2±254.2 (700.7–1091.6) | 1032.8±122.9 (944.8–1.120.7) | 19 |
| Total | 12 | 14 | |
95%CI: 95% confidence interval of the mean; SD: standard deviation. Nonsignificant (Mann–Whitney U-test). ADC units: mm2/s×10−6.
The ADC values were higher in patients with contusions than in those with other types of injuries. In patients with a poor prognosis, the ADC values were lower.
Discussion- 1.
The diffusion of water in the brain tissues, measured by MRI techniques, was greater in contusions than in other types of injuries, which could be an expression of vasogenic edema.
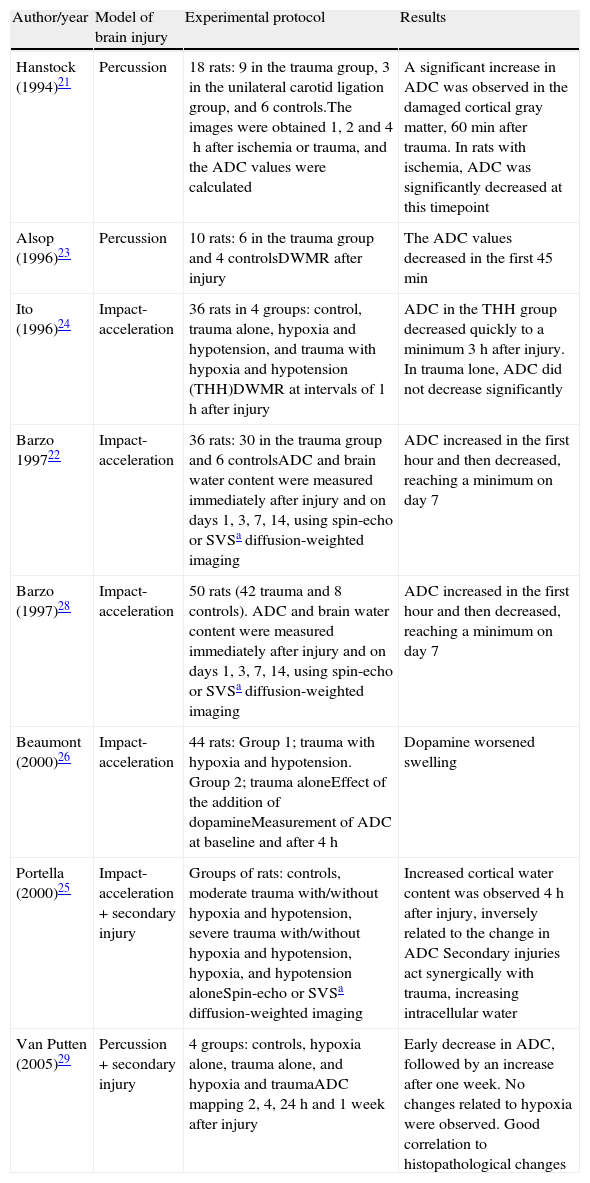
Findings of other studies: regarding the changes in ADC after traumatism, experimental studies in animals have yielded contradictory results, as can be seen in Table 6, where some authors have reported an increase in diffusion as a result of vasogenic edema21 while others describe a two-phase response22 or even significantly diminished diffusion values.23 Some studies only report changes when secondary damage is added, with diffusion decreasing as a result of cytotoxic edema.24–29
Table 6.Studies in animal models of brain injury.
Author/year Model of brain injury Experimental protocol Results Hanstock (1994)21 Percussion 18 rats: 9 in the trauma group, 3 in the unilateral carotid ligation group, and 6 controls.The images were obtained 1, 2 and 4h after ischemia or trauma, and the ADC values were calculated A significant increase in ADC was observed in the damaged cortical gray matter, 60min after trauma. In rats with ischemia, ADC was significantly decreased at this timepoint Alsop (1996)23 Percussion 10 rats: 6 in the trauma group and 4 controlsDWMR after injury The ADC values decreased in the first 45min Ito (1996)24 Impact-acceleration 36 rats in 4 groups: control, trauma alone, hypoxia and hypotension, and trauma with hypoxia and hypotension (THH)DWMR at intervals of 1h after injury ADC in the THH group decreased quickly to a minimum 3h after injury. In trauma lone, ADC did not decrease significantly Barzo 199722 Impact-acceleration 36 rats: 30 in the trauma group and 6 controlsADC and brain water content were measured immediately after injury and on days 1, 3, 7, 14, using spin-echo or SVSa diffusion-weighted imaging ADC increased in the first hour and then decreased, reaching a minimum on day 7 Barzo (1997)28 Impact-acceleration 50 rats (42 trauma and 8 controls). ADC and brain water content were measured immediately after injury and on days 1, 3, 7, 14, using spin-echo or SVSa diffusion-weighted imaging ADC increased in the first hour and then decreased, reaching a minimum on day 7 Beaumont (2000)26 Impact-acceleration 44 rats: Group 1; trauma with hypoxia and hypotension. Group 2; trauma aloneEffect of the addition of dopamineMeasurement of ADC at baseline and after 4h Dopamine worsened swelling Portella (2000)25 Impact-acceleration+secondary injury Groups of rats: controls, moderate trauma with/without hypoxia and hypotension, severe trauma with/without hypoxia and hypotension, hypoxia, and hypotension aloneSpin-echo or SVSa diffusion-weighted imaging Increased cortical water content was observed 4h after injury, inversely related to the change in ADC Secondary injuries act synergically with trauma, increasing intracellular water Van Putten (2005)29 Percussion+secondary injury 4 groups: controls, hypoxia alone, trauma alone, and hypoxia and traumaADC mapping 2, 4, 24h and 1 week after injury Early decrease in ADC, followed by an increase after one week. No changes related to hypoxia were observed. Good correlation to histopathological changes The apparently conflicting results of these studies could be attributed to the diversity of lesion models used, the different MRI techniques employed for measurement, and the lack of distinction between cortical lesions and diffuse axonal injury, which could involve different mechanisms and evolutive patterns. In animal studies, the ADC values are generally measured in the first minutes or hours after injury, while in humans ADC is measured from hours to days after trauma in many cases. This could explain the different ADC variation profiles observed.
Human studies have focused on three aspects: the identification of diffuse axonal injury and its diffusion characteristics; analysis of the type of edema in the brain lesions; and evaluation of the usefulness of DWMR in predicting the clinical outcome. Many authors agree that DWMR is more sensitive than other conventional MRI techniques in detecting DAI,30–32 but the observed diffusion changes vary. In a study of 116 patients with DAI, 64% of the lesions had diminished ADC values while 34% presented increased values.31 Liu reported decreased diffusion values in 9 patients with DAI. In these patients, the ADC values decreased significantly in typical DAI observed in conventional MRI images, from as early as one day after injury, and this decrease persisted for up to 18 days.33 On the other hand, the significance of these changes is also controversial. In this context, some authors consider that lesions with low ADC values are indicative of severe brain damage and could predict the long-term outcome.30,32 Schaefer et al.32 studied 26 patients with DAI using DWMR. They observed a strong correlation between the volume of the signal intensity alteration in the diffusion-weighted images and initial clinical severity, but this correlation did not improve on only considering lesions with decreased ADC values. Hou et al.34 in turn analyzed the changes in diffusion in 37 adults with brain injuries. Patients with more serious injuries were found to have ADC values significantly different from those of the controls, and patients with an unfavorable prognosis presented significantly higher ADC values than individuals with a favorable prognosis or the controls.
In our series we identified 7 cases of DAI, with mean ADC values above normal. After 6 months, three of these patients had a poor prognosis.
Regarding the type of edema, some authors consider that the distinction between intra- and extracellular edema is of key prognostic and therapeutic importance.35–37 In a series of patients with traumatisms in which the fluid content and ADC were measured using MRI, the authors observed an increase in tissue fluid associated with a decrease in ADC–thus suggesting predominant intracellular edema,35 though in this study the type of lesion and the time elapsed between trauma and MRI were not specified. In a later study, the same group36 used MRI techniques to identify the type of edema that developed in 44 patients with serious brain injuries. The patients with brain swelling showed an increased brain water content and low ADC values, compared with the healthy volunteers. The authors concluded that traumatic brain swelling appears to be predominantly cellular, as suggested by the low ADC values and the high tissue water content.
Our patients predominantly presented mass-type lesions, and the latter (12 non-evacuated, mixed-density contusions) showed an increase in diffusion that could be an expression of vasogenic edematization, even when only considering the images obtained in the first 48h after injury. Such lesions would correspond to type I injuries according to the classification proposed by Gasparetto,4 referred to traumatic brain injury patterns in DWMR/ADC imaging. According to this classification, type I injuries consist of hyperintense images in diffusion-weighted imaging with an increase in ADC, representing vasogenic edema. Type II injuries in turn comprise hyperintense lesions in diffusion but with a decrease in ADC, indicating cytotoxic edema, while type III injuries correspond to central hemorrhagic lesions surrounded by a hyperintense area in the diffusion image and an increase in ADC. It is important to note that in diffusion imaging of hemorrhagic lesions it is not clear whether low ADC values correspond to cytotoxic edema or to artifacts produced by extravasated blood.
- 2.
Our patients with a poor outcome had lower ADC values than those with a good outcome. Many studies have described different ADC values in patients with a poor prognosis compared with controls or patients with a good prognosis, but there is little consensus regarding the pattern of change, as reflected by the abovementioned studies.31,32,34,36–39
- 3.
Study limitations.
Of all the severe brain injury patients admitted to the ICU, we were only able to use DWMR in 23 cases, due to problems related to critical patient transfer, which make it difficult to perform early and repeated MRI scans. As a result, our sample size was small and scanning covered a broad time interval.
In addition, we had no cases of diffuse swelling for comparison with other types of injuries; as a result, our study fundamentally centers on focal injuries.
Another recognized limitation is the use of reference values cited in the literature, instead of values referred to contemporaneous controls.
The mean ADC values in the lesions were greater than the normal values and greater in contusions than in other types of injuries, thus indicating that extracellular edema probably plays a dominant role in these lesions.
The ADC values were decreased in patients with a poor prognosis, suggesting an association between ischemia and patient outcome.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Prieto-Valderrey F, Muñiz-Montes JR, López-García JA, Villegas-del Ojo J, Málaga-Gil J, Galván-García R. Utilidad de la resonancia magnética potenciada en difusión en pacientes con lesiones focales por traumatismo craneoencefálico grave. Med Intensiva. 2013;37:375–382.