The process of weaning from invasive mechanical ventilation is one of the central axes of the intensivists’ routine clinical practice, which can account for up to 40% of the overall time on invasive mechanical ventilation (IMV).1 It consists of 2 main phases: early detection of patients eligible to initiate it (screening), and confirmation test through the spontaneous breathing trial (SBT). Table 1.
Current standard of the weaning process.1
| Current standard of screening |
| 1. Subjective criteria: |
| • Resolution or stabilization of the condition that prompted IMV. |
| • Minimum level of continuous sedation. |
| • Respiratory rate > 6 bpm and ≤ 35 bpm. |
| 2. Objective criteria: |
| • Cardiovascular stability with a maximum of 0.1 mcg/kg/min of noradrenaline. |
| • Hemoglobin > 7 g/dL. |
| • Ion levels within range. |
| • Temperature within the range of 36–38.5 °C. |
| • Early predictive criteria of tolerance to SBT (RSBI, P0.1, MIP). |
| • PaO2/FiO2 ≥ 150 with PEEP ≤ 8 cm H2O. |
| Intolerance to SBT |
| 1. Subjective criteria: |
| • Decreased level of consciousness, profuse sweating, cyanosis, increased respiratory effort, and dyspnea. |
| 2. Objective criteria: |
| • PO2 ≤ 50–60 mm Hg with FiO2 ≥ 0.5 or SpO2 < 90%. |
| • PCO2 ≥ 50 mm Hg or increase ≥ 8 mm Hg from baseline. |
| • pH < 7.32 or decrease of ≥0.07 pH units from baseline. |
| • RR/TV (RSBI) > 105 breaths/min/L. |
| • Respiratory rate ≥ 35 bpm or 50% increase from previous. |
| • Heart rate ≥ 140 bpm, 20% increase, or arrhythmia. |
| • Systolic blood pressure < 90 mm Hg or ≥180 mm Hg or 20% increase. |
FiO2, fraction of inspired oxygen; MIP, maximal inspiratory pressure; P0.1, airway occlusion pressure in the first 0.1 s; PaO2, arterial oxygen pressure, PEEP, positive end-expiratory pressure; RR, respiratory rate; RSBI, Rapid Shallow Breathing Index; TV, tidal volume.
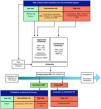
In recent years, 2 new aspects have been developed: risk stratification for extubation failure and the application of non-invasive respiratory support after extubation. This new approach requires dealing with 4 aspects simultaneously (stratification, screening, SBT, and post-extubation support), instead of the traditional approach of just 2 sequential phases. Fig. 1.
Proposal to individualize weaning considering individual risk stratification and post-extubation support application. BMI, body mass index; HFOT, high-flow oxygen therapy; NIV, non-invasive ventilation; PEEP, positive end-expiratory pressure; PS, pressure support; SBT, spontaneous breathing trial. *Aggressive screening5 **Conservative screening.1
To determine the individual risk of failure, multiple described factors exist (Table 2). The use of a complex model including all variables2 allows for better risk sub-stratification and subgroup detection, while a simple model with 3 variables only (age, chronic cardiac or pulmonary disease) (3) is pragmatic and reduces the burden of clinical work. It is still to be determined whether a 4-factor model, adding prolonged mechanical ventilation (>7 days of IMV) could be the optimal strategy in risk stratification.
Risk factors associated with failed extubation procedures.2,3
| 3-factor model | 11-factor model |
|---|---|
| Age > 65 years | Age > 65 years |
| Chronic heart disease | Heart failure as the reason for intubation |
| Chronic respiratory disease | Moderate-to-severe COPD |
| APACHE II score > 12 on extubation day | |
| BMI > 30 kg/m2 | |
| Secretion aspiration > 2 times within the 8 h prior to extubation | |
| Charlson comorbidity index > 1 (includes respiratory and cardiac comorbidities) | |
| Upper airway-related risk factors | |
| >1 failed SBT | |
| Development of hypercapnia at the end of SBT | |
| >7 days on IMV |
APACHE II, Acute Physiology and Chronic Health Evaluation; BMI, body mass index; COPD, chronic obstructive pulmonary disease; IMV, invasive mechanical ventilation. SBT, spontaneous breathing trial.
Non-invasive ventilatory support after extubation, either with facilitative or preventive intent, should be individualized based on individual risk and the presence of specific risk factors, given the differences in efficacy reported. Our recommendation with preventive intent includes the use of a 24-h course of high-flow oxygen therapy (HFOT) in low-risk patients (patients without any of the described risk factors), in intermediate-risk patients (≤3 risk factors excluding obese, hypercapnic patients after SBT), the combination of non-invasive mechanical ventilation (NIV) plus HFOT for 48 h,3 and in high-risk patients (≥4 risk factors, obese or hypercapnic after SBT), optimized NIV with gas conditioning at intermediate temperature (29 °C) for 48 h and selection of the appropriate interface for conditioning.2 Non-invasive ventilatory support with facilitative intent also requires introducing changes to screening and SBT.
The early detection of patients ready to start weaning from IMV is key to avoid any weaning delays. The screening process requires updating for several reasons. First, current recommendations applied to low or intermediate risk patients may result in unnecessary weaning delays, associated complications (e.g., acquired delirium at the ICU setting), and possible higher rate of failed SBT.4,5
Second, predicting SBT success using parameters such as rapid and shallow breathing index should not be confused with extubation success prediction. Third, too demanding non-respiratory screening criteria such as the use of noradrenaline at doses < 0.1 mcg/kg/min4,6,7 and inadequate sedation protocols may contribute to delays and increase result heterogeneity. Additionally, regarding the use of inotropes, no specific recommendations have been made despite being drugs that may be useful in transitioning to negative pressure spontaneous ventilation in patients with cardiac dysfunctions.
Fourth, the assessment of pulmonary function recovery has shown limited advances until recently, transitioning from PaO2/FiO2 > 200 with PEEP ≤ 5 cm H2O and FiO2 ≤ 40% to PaO2/FiO2 ≥ 150 with PEEP ≤ 8 and FiO2 ≤ 40% and finally to PaO2/FiO2 > 180 with PEEP 10 and FiO2 50%.5,6 Additionally, non-invasive therapies after extubation (whether preventive or facilitative) reduce respiratory effort, allowing changes to these screening parameters.
Diagnosis should be confirmed by analyzing performance during SBT, with 30 min of pressure support application, although the pressure range is still very wide (from 5 to 8 cm H2O).7 Therefore, the role of PEEP during SBT is still to be elucidated and is currently under study (Clinicaltrials.gov NCT 05526053). Also, epidemiological studies warn of the frequency of direct extubation without any confirmation tests being performed.4 Although this practice may shorten time on IMV, its use should be limited to very patients at very low risk of experiencing extubation failure until this practice is standardized or times are shortened by optimizing screening and SBT.
It seems imperative to individualize SBT based on each patient’s individual risk, screening, and therapy after extubation, rather than extubating without individualizing SBT or extubating specific subgroups after failed SBT to facilitative NIV, such as selected patients with hypoxemic failure8 or patients with COPD and hypercapnic failure.9
We face the need for redefining the weaning process. The application of non-invasive ventilatory support after extubation implies redefining terms such as reintubation, as it may delay the onset of respiratory failure after extubation.2 At the other end of the spectrum, the problem of accelerating extubation is raised. This is an excessively broad concept, as it includes various scenarios: aggressive screening without performing SBT, risk stratification, or prevention4; aggressive screening with intolerable SBT and application of facilitative NIV10; aggressive screening without performing SBT and application of facilitative NIV8; aggressive screening, tolerated conservative SBT with risk stratification and preventive HFOT.5 To date, the latter is the only one of these approaches tested with a design that reduces subjectivity with demonstrated clinical benefits.
In medicine, most diagnostic processes include screening and a confirmation test, and weaning should comply to the usual rules of medical diagnostic practice. Additionally, the evolution of medicine towards personalization should include weaning with the detection of subgroups with different clinical behavior.
Conflicts of interestGonzalo Hernández declares personal fees and travel expenses from Fisher & Paykel.