West Nile virus (WNV), an RNA virus of the genus Flavivirus, family Flaviviridae,1,2 is a zoonotic arbovirus transmitted by a mosquito of the genus Culex,2,3 and has a considerable worldwide public health impact.4
It was isolated for the first time in 1937 from a woman living in the West Nile region, in Uganda, and since then cases have been reported all over the world,5–7 including wide regions in western Europe.8 In Spain, the infection was confirmed in 2004, and since then cases have been reported mainly in Andalusia.3
In late July 2020, a WNV encephalitis epidemic was declared in the provinces of Seville, Cádiz and Badajoz. The Spanish National Center of Epidemiology was informed of 69 cases of WNV fever (35 confirmed and 34 probable): 56 in the province of Seville, 12 in Cádiz and one Badajoz – with 41 hospital admissions during the epidemic, 17 admissions to the Intensive Care Unit (ICU) and 7 deaths.9
Hospital Virgen del Rocío assisted 35 patients, with 9 individuals requiring admission to the ICU because of severe encephalitis. All the patients lived in the geographical setting of the Guadalquivir river basin, in the towns of Coria and Puebla del Río. No common exposures of the patients were identified, though all the affected individuals were involved in open-air activities. The demographic data are reported in Table 1.
Demographic characteristics and hospital course of the patients admitted to the ICU due to West Nile virus infection in the region of the Guadalquivir river basin.
| Mean | n | Range/percentage | |
|---|---|---|---|
| Median age (years) | 66 | 14−77 | |
| Gender | |||
| Female | 4 | 44.4 | |
| Male | 5 | 55.6 | |
| Age distribution (years) | |||
| 10−20 | 1 | 11.1 | |
| 50−60 | 1 | 11.1 | |
| Over 60 | 7 | 77.7 | |
| Personal history | 7 | 77.7 | |
| Patients with PDH | 5 | 55.6 | |
| AHT | 3 | 33.3 | |
| DM II | 3 | 33.3 | |
| Lung disease | 1 | 11.1 | |
| CKD | 1 | 11.1 | |
| CVA | 1 | 11.1 | |
| Liver transplantation | |||
| Days from symptoms onset to ICU admission | 5 | 3−7 | |
| GCS upon admission to ICU | 8 | 6−11 | |
| GCS at discharge from ICU | 12 | 8−15 | |
| Days of ICU stay | 18 | 4−30 | |
| Diagnosis | |||
| IgM | 8 | 88.8 | |
| CRP in urine | 1 | 11.1 | |
| Brain CT | 9 | 100 | |
| Normal | 6 | 66.7 | |
| Evidence of acute process | 0 | 0 | |
| Atrophy or chronic ischemic changes | 3 | 33.3 | |
| Brain MRI | 6 | 66.6 | |
| Normal | 3 | 33.3 | |
| Evidence of acute process | 2 | 22.2 | |
| Atrophy or ischemic changes | 1 | 11.1 | |
| EEG | 7 | 77.8 | |
| Normal | 4 | 44.4 | |
| Pathological | 3 | 33.3 | |
| EPS | 3 | 33.3 | |
| Normal | 0 | 0 | |
| Pathological | 3 | 33.3 | |
| Treatment | |||
| None | 2 | 22.2 | |
| Corticosteroids | 7 | 77.8 | |
| Immunoglobulin | 1 | 11.1 | |
| Tracheostomy | 6 | 66.6 | |
| Death in ICU | 2 | 22.2 | |
| Death during first year | 3 | 33.3 | |
| Full recovery | 3 | 33.3 | |
| Major disability | 1 | 11.1 |
PDH, previous disease history; CVA, cerebrovascular accident; DM II, type II diabetes mellitus; GCS, Glasgow Coma Score; EPS, electrophysiological study; EEG, electroencephalographic study; CKD, chronic kidney disease; AHT, arterial hypertension; IgM, immunoglobulin M; n, number de cases; CRP, C-reactive protein; MRI, magnetic resonance imaging; CT, computed tomography; ICU, Intensive Care Unit.
The reason for ICU admission was impaired consciousness and respiratory failure in all cases. The mean duration of the symptoms before hospital admission was four days, versus 5 days until ICU admission. All of the patients suffered severe neurological deterioration, with a mean Glasgow Coma Score (GCS) of 8 points (range 6−11). The most common symptom was fever (90%), followed by weakness (88%), neck stiffness (44%), vomiting (33%), headache (33%) and diplopia (22%). Muscle involvement was very prevalent, with extrapyramidal symptoms in 66% of the cases, flaccid paralysis with lower limb weakness in 55%, spastic muscle stiffness in 44% and hyporeflexia in 32%.
The blood count showed variable degrees of leukocytosis, with a mean count of 12,787/mL (interquartile range [IQR] 7020–20,900); there were no erythrocyte or platelet alterations. Two patients suffered acute renal failure and another two developed liver alterations.
The cerebrospinal fluid (CSF) puncture findings were suggestive of viral infection, showing pleocytosis with a mean leukocyte count of 257/mm3 (IQR 14-1275): 66.6% lymphocytes and 33.4% neutrophils. Cerebrospinal fluid protein elevation was observed, with a mean concentration of 97.56mg/dl (IQR 38-899). None of the patients presented diminished CSF glucose levels.
A FilmArray® study was made in CSF, involving polymerase chain reaction testing for the most common pathogens causing community-acquired meningoencephalitis.10 The results proved negative in all cases. The CSF, serum and urine samples were sent to the National Microbiological Research Center in Granada, where they underwent indirect IgM and IgG capture ELISA testing, as well as real-time polymerase chain reaction (PCR) assay to detect antibodies against WNV. Confirmation diagnosis was performed in 100% of the patients: 8 involving IgM serological testing and one based on urine PCR testing.
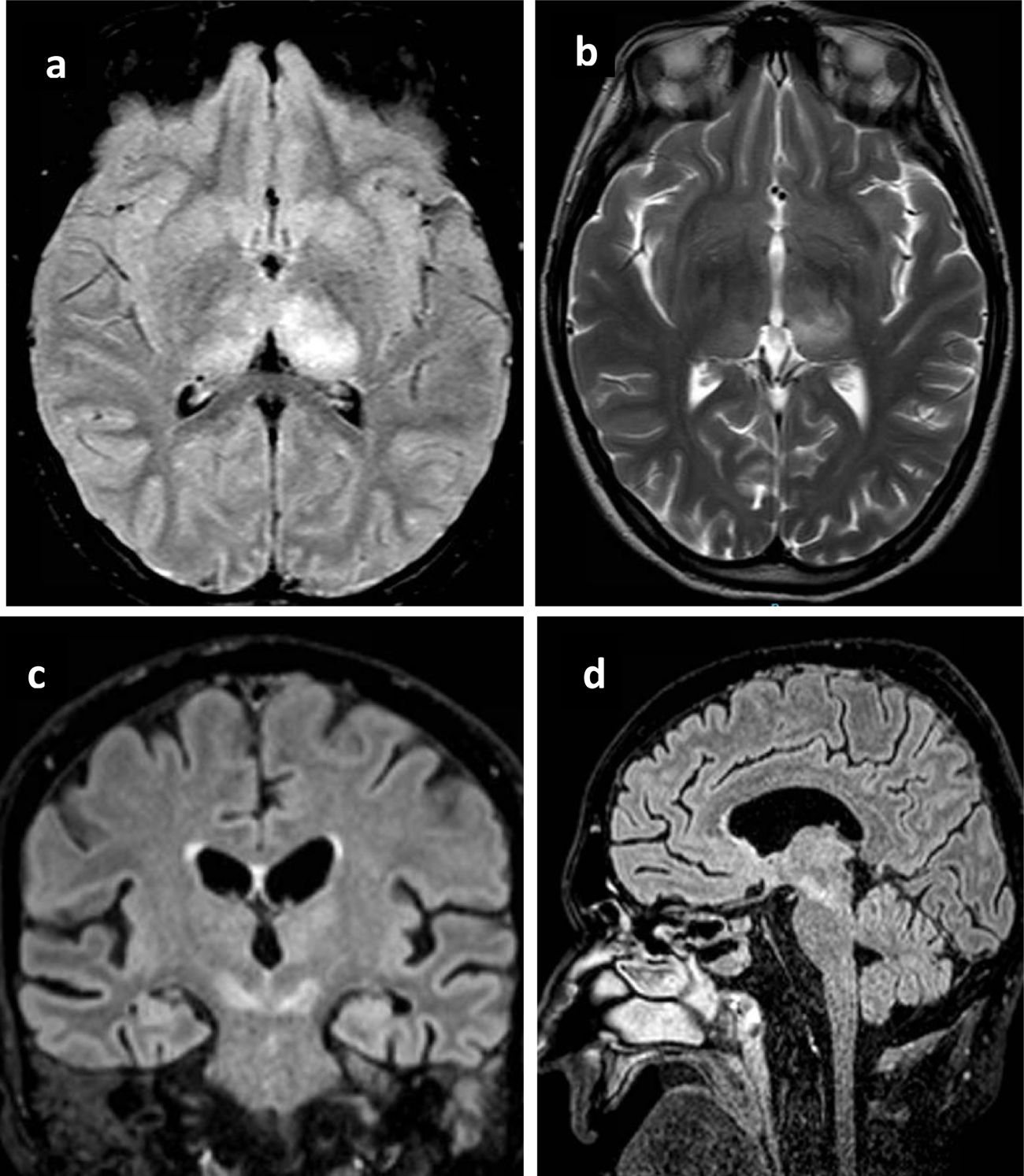
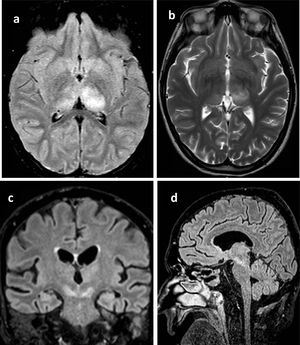
All patients underwent brain computed tomography (CT) evaluation, with no acute alterations being observed in any of them. In view of the presence of neurological clinical manifestations without CT abnormalities, brain magnetic resonance imaging (MRI) was requested in 6 patients, with the identification of structural lesions in three of them (Fig. 1).
Principales hallazgos en RMN en pacientes con infección por Virus del Nilo Occidental. Paciente 1: a) Cambios en la intensidad de señal en el margen posterior de ambos tálamos, fundamentalmente el izquierdo con hipointensidad heterogénea en T1. b) hiperintensidad heterogénea en secuencia T2 en ambos tálamos principalmente izquierdo. Paciente 2: c) alteración del tronco del encéfalo con afectación hiperintensa marcada en banda en ambos pedúnculos cerebrales del mesencéfalo. Paciente 3: d) Lesión hiperintensa en la línea media del mesencéfalo que se extiende caudal hasta la protuberancia.
An electroencephalographic (EEG) study was made in 7 patients, of which four showed a moderate diffuse cerebral dysfunctional pattern – with no epileptic activity in any case. In turn, an electrophysiological study was carried out in three subjects, with the following findings: (1) peripheral nervous system involvement with severely decreased motor and sensory amplitudes and active denervation on the electromyogram; (2) predominantly motor axonal polyneuropathy with preservation of the sensory responses secondary to the possible involvement of the anterior horn of the spinal cord; and (3) altered auditory evoked potentials due to brainstem involvement.
Following admission to the ICU, all 9 patients required some ventilatory support measure, 7 required orotracheal intubation, and two needed high-flow oxygen with a nasal cannula. Vasoactive hemodynamic support proved necessary in 7 patients. None of the subjects required renal replacement therapy.
Methylprednisolone pulses were administered for 5 days in 8 patients. This was done early (within <24h after admission to the ICU) in 5 patients, of which three presented a favorable neurological course. One patient received human immunoglobulin, with an unfavorable neurological outcome. In this case treatment was carried out late (after 18 days), due to delays in the microbiological diagnosis. At present, and even though various drugs were used to treat the WNV infection (corticosteroids, ribavirin, immunoglobulin, aciclovir, interferon), none proved to be effective.1–3
The clinical course was variable (Table 1). The progressive and severe neurological deterioration, with variable GCS scores between 3 and 9 points, implied the need for orotracheal intubation in 7 patients. Those who did not require orotracheal intubation presented a favorable course, with early recovery of the GCS score to 14–15 points. The youngest patient showed a torpid clinical course, with recovery of the level of consciousness, left-side hemiplegia and right-side motor involvement with flaccid paralysis. Three patients evolved towards a vigil coma state, requiring tracheostomy; weaning from mechanical ventilation was achieved, and the patients were moved to the hospital ward. Two patients suffered rapidly progressing neurological deterioration, with severe brainstem involvement, and died during admission to the ICU.
Following discharge from the ICU, the three patients that evolved towards vigil coma without consciousness had died, with a mean survival of 2.1 months. The youngest patient (a minor) continued to present an important neurological deficit, though he was conscious, and the three patients that evolved favorably in the ICU recovered functional autonomy with no major neurological sequelae at one year of follow-up.
In conclusion, WNV infection in our setting must be taken into account in the differential diagnosis of viral meningoencephalitis. Neurological involvement is usually severe, and diagnostic confirmation tends to be established late. These two factors, together with the lack of effective treatment, imply that management in the ICU essentially focuses on life support, with poor clinical outcomes in the form of neurological damage and death. Faster diagnostic techniques and effective treatments are needed to act upon the virus or attenuate the brain damage, with a view to improving the patient prognosis.
Ethics Committee approvalThe present study was evaluated and approved by the Ethics and Biomedical Research Portal of Andalusia, with designation of the Research Ethics Committees of Hospital Universitario Virgen Macarena and Hospital Universitario Virgen del Rocío (internal code: 0733-N-21), which issued a favorable opinion on 26 October 2021.
Special recognition must go to the patients and families affected by the WNV encephalitis outbreak, and particularly to those who died of the disease. We hope that preventive strategies and adequate treatment will soon be available, allowing us to avoid severe involvement as a result of this disorder.
We thank the healthcare professionals of the ICU of Hospital Universitario Virgen del Rocío, who dedicate great effort every day to the care of their patients, offering them the best conditions for overcoming their illnesses.
Likewise, thanks are due to the entire staff of Hospital Universitario Virgen del Rocío related to the diagnosis and management of patients in the emergency care setting, hospital wards and microbiology laboratory, since their contributions made it possible to offer timely care for our patients.
Please cite this article as: Cuenca-Apolo, García-Delgado Rosado H, Amaya Villar R, Brote de encefalitis por virus del Nilo Occidental en el área de la cuenca del río Guadalquivir: Experiencia y resultados en los pacientes ingresados en cuidados intensivos, Medicina Intensiva. 2022;46:530–533.