To assess the clinical profile and factors associated with 30-day mortality in patients with acute heart failure (AHF) admitted to the intensive care unit (ICU).
DesignProspective, multicentre cohort study.
ScopeThirty-two Spanish ICUs.
PatientsAdult patients admitted to the ICU between April and June 2017.
InterventionPatients were classified into three groups according to AHF status: without AHF (no AHF); AHF as the primary reason for ICU admission (primary AHF); and AHF developed during the ICU stay (secondary AHF).
Main variables of interestIncidence of AHF and 30-day mortality.
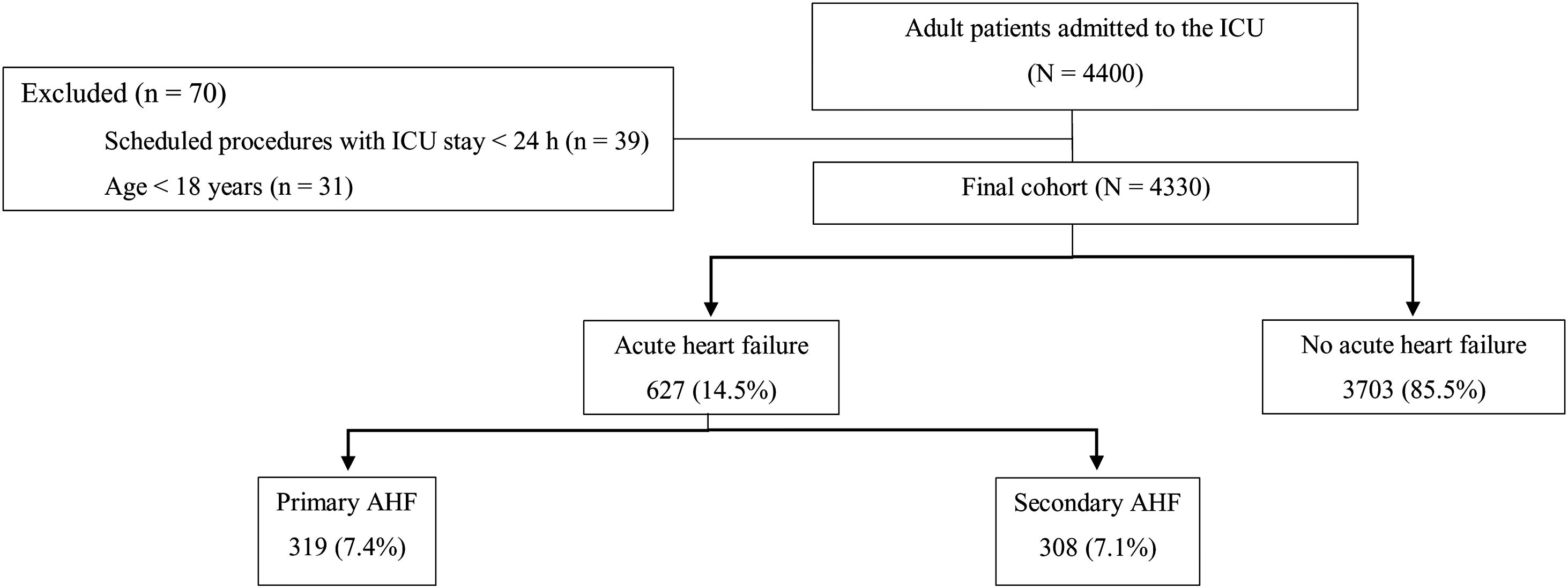
ResultsA total of 4330 patients were included. Of these, 627 patients (14.5%) had primary (n=319; 7.4%) or secondary (n=308; 7.1%) AHF. Among the main precipitating factors, fluid overload was more common in the secondary AHF group than in the primary group (12.9% vs 23.4%, p<0.001). Patients with AHF had a higher risk of 30-day mortality than those without AHF (OR 2.45; 95% CI: 1.93–3.11). APACHE II, cardiogenic shock, left ventricular ejection fraction, early inotropic therapy, and diagnostic delay were independently associated with 30-day mortality in AHF patients. Diagnostic delay was associated with a significant increase in 30-day mortality in the secondary group (OR 6.82; 95% CI 3.31–14.04).
ConclusionsThe incidence of primary and secondary AHF was similar in this cohort of ICU patients. The risk of developing AHF in ICU patients can be reduced by avoiding modifiable precipitating factors, particularly fluid overload. Diagnostic delay was associated with significantly higher mortality rates in patients with secondary AHF.
Evaluar el perfil clínico y los factores asociados con la mortalidad a 30 días en pacientes con insuficiencia cardíaca aguda (ICA) ingresados en Unidades de Cuidados Intensivos (UCI).
DiseñoProspectivo, multicéntrico.
Ámbito32 UCI españolas.
PacientesPacientes adultos ingresados en UCI entre abril y junio de 2017.
IntervenciónLos pacientes se clasificaron en tres grupos según el estado de la ICA: sin ICA (no ICA), ICA como motivo principal de ingreso en UCI (ICA-primaria), e ICA desarrollada durante la estancia en UCI (ICA-secundaria).
Principales variables de interésIncidencia de ICA y mortalidad a los 30 días.
ResultadosSe incluyeron 4.330 pacientes, de estos, 627 (14,5%) tenían ICA-primaria (n = 319; 7,4%) o secundaria (n = 308; 7,1%). Entre los principales factores precipitantes, la sobrecarga hídrica fue más común en el grupo ICA-secundaria que el ICA-primaria (12,9 vs. 23,4%, p < 0,001). Los pacientes con ICA tuvieron un mayor riesgo de mortalidad que los que no tenían ICA (OR 2,45; IC 95%: 1,93-3,11). APACHE II, choque cardiogénico, fracción de eyección del ventrículo izquierdo, tratamiento precoz con inotrópicos y el retraso diagnóstico se asociaron de forma independiente con la mortalidad en los pacientes con ICA. El retraso diagnóstico se asoció con un aumento significativo de mortalidad en el grupo secundario (OR 6,82; IC 95%: 3,31-14,04).
ConclusionesLa incidencia de ICA primaria y secundaria fue similar. El riesgo de desarrollar ICA en pacientes críticos puede reducirse evitando factores precipitantes modificables, en particular la sobrecarga de líquidos. El retraso diagnóstico se asoció con mayor mortalidad en pacientes con ICA-secundaria.
Heart failure is a clinical syndrome characterized by a set of clinical signs (elevated jugular venous pressure, pulmonary congestion) and non-specific symptoms (dyspnea, orthopnoea, lower limb swelling) caused by a structural and/or functional cardiac abnormality, leading to reduced cardiac output and/or elevated intracardiac pressures at rest or during stress.1 The number of patients affected by this condition is expected to increase over time due to improved treatment of acute cardiovascular disease, together with population aging.2
Acute heart failure (AHF) is a life-threatening condition characterized by rapid onset or worsening of symptoms and/or signs of heart failure.3 AHF requires urgent evaluation and treatment, typically necessitating admission to the intensive care unit (ICU) for organ support or monitoring. AHF can be present as a first occurrence or as a consequence of acute exacerbation of chronic heart failure due to extrinsic factors that are common in ICU patients (e.g., infection, stress, discontinuation of chronic medication, positive fluid balance).4,5 AHF can be the main factor leading to ICU admission, as well as a secondary medical condition that may emerge during the ICU stay, thus worsening the prognosis of critically ill patients.6
Data on AHF remain scant, particularly in critically ill patients.7–10 Given the need for more data in this clinical setting, we designed a nationwide, pragmatic real-life/bedside study in ICUs in Spain. The main aims of this study were: (1) to assess the incidence and management of patients with primary AHF (defined as the primary reason for ICU admission) and secondary AHF (defined as AHF developed during the ICU stay) and (2) to assess 30-day mortality according to type of AHF (primary vs. secondary) and to determine the variables independently-associated with these outcome measures.
Patients and methodsPatients and data collectionThis was a prospective, multicentre cohort study conducted in 32 ICUs in Spain. The size and medical activity of the participating centres varied significantly, representing the range of real-life variation in Spanish ICUs. The study protocol was developed with the support of the Spanish Society of Intensive Care Medicine and Coronary Units (SEMICYUC) and approved by the ethics committees at all participating hospitals.
From April to June 2017, all adult patients (≥ age 18) admitted to the ICUs at the participating centres for any acute condition were consecutively enrolled in the study. Patients younger than age 18 and those undergoing scheduled procedures with an ICU stay<24h were excluded. Demographic data, reason for admission, illness severity (APACHE II score), and Charlson comorbidity index from all patients were recorded in an online database.
Since this was a “real-life” study, the diagnosis of AHF was made by the treating physician according to his/her clinical judgment based on signs, symptoms and complementary testing. The lead investigator at each centre reviewed the clinical records to determine whether AHF was recorded as a final diagnosis at ICU discharge.
Patients were classified into three groups, as follows: no AHF, primary AHF (main reason for ICU admission), or secondary AHF (developed during the ICU stay). Patients classified with primary AHF could not be further classified into the secondary group.
Diagnostic delay was recorded as a dichotomous variable and defined as>12h between the onset of signs and symptoms and the diagnosis of AHF. The 12-h cut-off point was based on data reported in previous studies.11 Diagnostic delay was assessed by the main investigator at each centre based on a review of clinical data and complementary tests. Treatment was considered early when started within the first 24h after diagnosis.
Data on precipitating factors, clinical manifestations, diagnostic tests, and treatments for heart failure were recorded at the time of diagnosis in patients admitted to the ICU for AHF as well as those who developed AHF during the ICU stay, regardless of whether the condition was de novo or acute decompensation of chronic heart failure. Pulmonary oedema was defined by the presence of signs and symptoms of AHF, respiratory failure, and radiological evidence of pulmonary congestion. Cardiogenic shock was defined as the presence of signs and symptoms of AHF and evidence of hypoperfusion. Both conditions were not mutually exclusive.
Diagnosis of sepsis/septic shock was made according to the Sepsis-3 criteria.12 Fluid overload was recorded as a dichotomous variable according to the clinical judgement of the main investigator at each centre after reviewing fluid balance records. Weaning-induced pulmonary oedema was diagnosed by the treating physician according to clinical criteria.6 Stress cardiomyopathy was defined as an acute, transient systolic dysfunction due to mental or physical stress, including sepsis-induced myocardial depression, in accordance with current clinical guidelines.13 Left ventricular systolic and diastolic function were evaluated according to the current guidelines.14,15
Quality controlData verification was performed by a steering committee comprised of three senior intensive care specialists who randomly selected 20% of patients diagnosed with AHF from each participating centre during the study period. To ensure the reliability of the study data, these patients were revaluated, and a concordance analysis (Cohen's kappa) was performed to confirm the diagnosis of AHF. If the concordance analysis revealed an excessive lack of agreement (kappa<0.8) between the initial diagnosis and the reevaluated diagnosis, the data from that centre were excluded. Consequently, of the 34 ICUs that initially agreed to participate, 32 were included in the final analysis.
Statistical analysisDue to the observational nature of the study, a formal calculation of the study sample size was not applicable.
Data are presented as numbers (%) for categorical data. Continuous variables are presented as means with 95% confidence intervals (CI) if normally distributed or medians with interquartile range (IQR) if non-normally distributed. Fisher's exact test was used to compare categorical data. For univariate comparisons of continuous variables, we used the Student's t, ANOVA, Mann–Whitney U, or Kruskal–Wallis tests as appropriate based on the data distribution. Statistical significance was set at p≤0.05.
Univariate analyses were performed to assess the demographic variables and clinical variables potentially associated with 30-day mortality. Independent factors were identified using Cox regression analysis. The following variables were entered into the model based on their clinical relevance in previous studies8,16,17: age and APACHE II score at ICU admission; presence of cardiogenic shock; administration of vasopressors or inotropes; left ventricular ejection fraction; need for invasive mechanical ventilation; and highly significant (p<0.005) variables from the univariate analysis. The IBM-SPSS statistical software program (v. 26) was used to perform all statistical analyses.
ResultsPatientsDuring the study period, 4400 patients were admitted to the 32 participating ICUs (Fig. 1). Of these patients, 70 did not meet the study inclusion criteria and were excluded, leaving a total of 4330 patients. Most participants (80%) were recruited from medical-surgical ICUs, followed by surgical ICUs (12%), and coronary units (8%). Of these 4330 patients, 627 (14.5%) were diagnosed with primary or secondary AHF, distributed as follows: primary AHF (n=319; 7.4%) and secondary AHF (n=308; 7.1%).
The baseline characteristics of the sample (overall and by AHF group) are shown in Table 1. Compared to the patients without AHF, those diagnosed with AHF (primary or secondary) were older (62 vs. 69 years), more severely unwell at admission (APACHE II score: 13 vs. 19) and had more comorbidities (Charlson comorbidity index: 2 vs. 4). There were no differences in baseline characteristics between the primary and secondary groups. Admission for non-ischemic heart disease (52.4%) was more common in the primary AHF group while sepsis (24.3%) was more common in the secondary AHF group.
Baseline characteristics of the total population and by AHF subgroup.
| Acute heart failure | Acute heart failure subgroups | |||||
|---|---|---|---|---|---|---|
| No | Yes | p value | Primary | Secondary | p value* | |
| n=3703 | n=627 | n=319 | n=308 | |||
| Male | 2320 (63%) | 391 (62%) | 0.889 | 199 (62%) | 192 (62%) | 0.991 |
| Age (years) | 62 (61–62) | 69 (67–70) | <0.001 | 69 (68–71) | 68 (66–69) | 0.175 |
| BMI (kg/m2) | 27.8 (27.5–28) | 28.6 (28.1–29.2) | 0.003 | 28.9 (28.1–29.6) | 28.3 (27.6–29.1) | 0.337 |
| APACHE II | 13 (13–14) | 19 (18–20) | <0.001 | 19 (18–20) | 19 (18–20) | 0.443 |
| Charlson comorbidity index | 2 (2–2) | 4 (3–4) | <0.001 | 4 (3–4) | 3 (3–4) | 0.435 |
| Comorbidities | ||||||
| Alcohol | 791 (21.4%) | 131 (20.9%) | 0.791 | 69 (21.6%) | 62 (20.1%) | 0.644 |
| Smoking | 1595 (43.1%) | 301 (48%) | 0.021 | 152 (47.6%) | 149 (48.4%) | 0.855 |
| Hypertension | 1778 (48%) | 419 (66.8%) | <0.001 | 220 (69%) | 199 (64.6%) | 0.247 |
| Diabetes | 833 (22.5%) | 266 (42.4%) | <0.001 | 143 (44.8%) | 123 (39.9%) | 0.215 |
| Dyslipidemia | 1264 (34.1%) | 315 (50.2%) | <0.001 | 165 (51.7%) | 150 (50.2%) | 0.449 |
| Cardiomyopathy | ||||||
| Ischemic | 435 (11.7%) | 182 (29%) | <0.001 | 102 (32%) | 80 (26%) | 0.098 |
| Valvular | 232 (6.3%) | 126 (20.1%) | <0.001 | 71 (22.3%) | 55 (17.9%) | 0.169 |
| Dilated | 60 (1.6%) | 63 (10%) | <0.001 | 37 (11.6%) | 26 (8.4%) | 0.189 |
| Hypertrophic | 110 (3%) | 56 (8.9%) | <0.001 | 27 (8.5%) | 29 (9.4%) | 0.676 |
| Chronic heart failure | 225 (6.4%) | 207 (33.4%) | <0.001 | 127 (40.1) | 80 (26.4%) | <0.001 |
| Chronic renal failure | 292 (7.9%) | 141 (22.5%) | <0.001 | 86 (27%) | 55 (17.9%) | 0.006 |
| COPD | 383 (10.3%) | 103 (16.4%) | <0.001 | 56 (17.6%) | 47 (15.3%) | 0.438 |
| Reason for ICU admission | ||||||
| Non-ischemic heart disease | 266 (7.2%) | 201 (32%) | <0.001 | 167 (52.4%) | 34 (11.1%) | <0.001 |
| Ischemic heart disease | 528 (14.3%) | 174 (27.7%) | <0.001 | 99 (31%) | 75 (24.4%) | 0.074 |
| Sepsis/septic shock | 375 (10.1%) | 79 (12.6%) | 0.067 | 6 (1.9%) | 73 (23.7%) | <0.001 |
| Respiratory | 167 (4.5%) | 44 (7%) | 0.007 | 23 (7.2%) | 21 (6.8%) | 0.877 |
| Decompensated COPD | 75 (2%) | 17 (2.7%) | 0.293 | 2 (0.6%) | 15 (4.8%) | 0.001 |
| Digestive | 148 (4%) | 8 (1.3%) | 0.001 | 1 (0.3%) | 7 (2.3%) | 0.027 |
| Neurologic | 465 (12.6%) | 15 (2.4%) | <0.001 | 2 (0.6%) | 13 (4.2%) | 0.018 |
| Polytrauma | 325 (8.8%) | 7 (1.1%) | <0.001 | 1 (0.3%) | 6 (1.9%) | 0.031 |
| Non-cardiac surgery | 892 (24.1%) | 21 (3.4%) | <0.001 | 2 (0.6%) | 19 (6.2%) | <0.001 |
| Cardiac surgery | 147 (4%) | 32 (5.2%) | 0.193 | 6 (1.9%) | 26 (8.4%) | <0.001 |
| Other | 315 (8.5%) | 29 (4.6%) | 0.001 | 10 (3.2%) | 19 (6.2%) | 0.668 |
BMI: body mass index; APACHE: Acute Physiology and Chronic Health Evaluation; CHF: chronic heart failure; COPD: chronic obstructive pulmonary disease; ICU: intensive care unit.
Data are presented as n (%) or means (95% confidence interval).
Table 2 shows the following: clinical findings, underlying cardiac disease, precipitating factors, tests performed at the time of diagnosis, and early interventions in patients with AHF. Pulmonary oedema was the most common form of presentation (78.5%). Although ischemic heart disease was the most common underlying disease in both groups, it was more prevalent in the primary group (42% vs. 32.5%). By contrast, stress cardiomyopathy was more prevalent in the secondary group (1.9% vs. 12.7%).
Clinical findings, underlying cardiac diseases, precipitating factors, tests performed at the time of diagnosis, and early interventions.
| Overall AHFn=627 | Primary AHFn=319 | Secondary AHFn=308 | p value* | |
|---|---|---|---|---|
| Symptoms/signs at onset | ||||
| Pulmonary edema | 492 (78.5%) | 275 (86.2%) | 217 (70.5%) | <0.001 |
| Cardiogenic shock | 268 (42.7%) | 150 (47%) | 118 (38.3%) | 0.028 |
| Underlying cardiac disease | ||||
| Ischemic heart disease | 234 (37.3%) | 134 (42%) | 100 (32.5%) | 0.014 |
| Cardiomyopathy | ||||
| Dilated | 81 (12.9%) | 56 (17.6%) | 25 (8.1%) | <0.001 |
| Hypertensive | 49 (7.8%) | 23 (7.2%) | 26 (8.4%) | 0.566 |
| Stress cardiomyopathy | 45 (7.2%) | 6 (1.9%) | 39 (12.7%) | <0.001 |
| Cor pulmonale | 36 (5.7%) | 16 (5%) | 20 (6.5%) | 0.426 |
| Hypertrophic | 30 (4.8%) | 16 (5%) | 14 (4.5%) | 0.783 |
| Restrictive | 4 (0.6%) | 2 (0.6%) | 2 (0.6%) | 0.972 |
| Arrhythmia | 192 (30.6%) | 116 (36.4%) | 76 (24.7%) | 0.002 |
| Valvulopathy | 138 (22%) | 94 (29.5%) | 44 (14.3%) | <0.001 |
| Other | 25 (3.9%) | 12 (6.3%) | 4 (3.1%) | 0.117 |
| Precipitating factors | ||||
| Not known | 321 (51.7%) | 191 (60%) | 130 (42.2%) | <0.001 |
| Sepsis/septic shock | 149 (23.7%) | 58 (18.2%) | 91 (29.5%) | 0.001 |
| Fluid overload | 113 (18%) | 41 (12.9%) | 72 (23.4%) | 0.001 |
| Renal failure | 93 (14.8%) | 46 (14.4%) | 47 (15.3%) | 0.767 |
| Anemia | 56 (8.9%) | 27 (8.5%) | 29 (9.4%) | 0.676 |
| Changes in chronic heart failure drugs | 50 (7.9%) | 24 (7.5%) | 26 (8.5%) | 0.669 |
| Weaning-induced pulmonary oedema | 26 (4.1%) | 1 (0.3%) | 25 (8.1%) | <0.001 |
| Diagnosis and tests performed | ||||
| Electrocardiogram | 561 (89.5%) | 299 (93.7%) | 262 (85.1%) | <0.001 |
| Chest X-ray | 561 (89.5%) | 301 (94.4%) | 260 (84.4%) | <0.001 |
| Troponin determination | 459 (73.2%) | 264 (82.8%) | 195 (63.3%) | <0.001 |
| Natriuretic peptides determination | 217 (34.6%) | 114 (35.7%) | 103 (33.4%) | 0.546 |
| Echocardiography | 460 (73.4%) | 253 (79.3%) | 207 (67.2%) | 0.001 |
| LVEF<50% (yes/reported) | 242/419 (57.7%) | 141/236 (59.7%) | 100/183 (54.6%) | 0.295 |
| LVEF (%) | 43 (42–45) | 42 (40–44) | 45 (43–47) | 0.022 |
| Diastolic dysfunction (yes/reported) | 30/75 (40%) | 20/43 (46.5%) | 10/32 (31.2%) | 0.182 |
| E/Ea | 12.7 (10.7–14.7) | 14.1 (11.1–17.1) | 10.8 (8.5–13.1) | 0.099 |
| Right ventricular dysfunction (yes/reported) | 106/243 (43.6%) | 57/138 (41.3%) | 49/105 (46.6%) | 0.404 |
| TAPSE (mm) | 17.9 (17.3–18.5) | 18.1 (17.3–18.9) | 17.6 (16.8–18.4) | 0.432 |
| Hemodynamic monitoring | 142 (22.6%) | 59 (18.5%) | 83 (26.9%) | 0.011 |
| Pulmonary artery catheter | 30 (4.8%) | 13 (4.1%) | 17 (5.9%) | 0.397 |
| Transpulmonary thermodilution | 29 (4.6%) | 9 (2.9%) | 20 (6.9%) | 0.029 |
| Pulse contour cardiac output analysis | 83 (13.8%) | 37 (11.6%) | 46 (14.9%) | 0.218 |
| Diagnostic delay | 74 (11.8%) | 33 (10.3%) | 41 (13.3%) | 0.158 |
| Interventions | ||||
| Oxygen | 549 (87.6%) | 293 (92%) | 256 (83%) | 0.001 |
| Morphine | 313 (49.9%) | 162 (50.8%) | 151 (49%) | 0.660 |
| Diuretic | 489 (78%) | 252 (79%) | 237 (76.9%) | 0.536 |
| Nitrates | 177 (28.2%) | 103 (32.3%) | 74 (24%) | 0.022 |
| Inotropes | 166 (26.5%) | 97 (30.4%) | 69 (22.4%) | 0.023 |
| Vasopressors | 238 (38%) | 117 (36.7%) | 121 (39.3%) | 0.501 |
| Antiarrhythmic | 148 (23.6%) | 81 (25.4%) | 67 (21.8%) | 0.283 |
| Beta blockers | 108 (17.2%) | 55 (17.2%) | 53 (17.2%) | 0.991 |
| Coronary angiography | 182 (29%) | 112 (35.1%) | 70 (22.7%) | 0.001 |
| Hemodynamic mechanical support | 18 (2.9%) | 12 (3.8%) | 6 (1.9%) | 0.174 |
| CPAP or NIV | 215 (34.3%) | 129 (40.4%) | 86 (27.9%) | 0.001 |
| Invasive mechanical ventilation | 210 (33.5%) | 106 (33.2%) | 104 (33.8%) | 0.887 |
| Renal replacement therapy | 55 (8.8%) | 30 (9.4%) | 25 (8.1%) | 0.569 |
AHF: acute heart failure; LVEF: left ventricular ejection fraction; E/Ea: ratio of transmitral early peak velocity (E) measured with pulsed wave Doppler to mitral annular early-diastolic peak velocity (Ea) measured with tissue Doppler; TAPSE: tricuspid annular plane systolic excursion; CPAP: continuous positive airway pressure; NIV: non-invasive ventilation.
Data are presented as n (%) or mean (95% confidence interval).
The main precipitating factor was unknown in 51.7% of patients. The following precipitating factors were all more common in the secondary group: infection (18.2% vs. 29.5%), fluid overload (12.9% vs. 23.4%) and weaning-induced pulmonary oedema (0.3% vs. 8.1%).
Electrocardiogram, chest X-ray, and troponin measurement were performed more frequently in the primary group. Natriuretic peptide levels were measured in 34.6% of patients, with no significant differences between groups. Echocardiography was performed in 73.4% of patients, but more commonly in the primary group (79.3% vs. 67.2%). Determination of left ventricular ejection fraction was reported in 419 of 460 echocardiograms, which was lower in the primary group (42% vs. 45%). Diastolic function was reported in 16.3% of echocardiograms performed. Minimally invasive and invasive hemodynamic monitoring was used in a higher proportion of the secondary group (18.5% vs. 26.9%). Overall, diagnosis of AHF was delayed in 11.8% of patients, with no significant differences between the primary and secondary groups (10.3% vs.13.3%, respectively).
The most common drug for early management were diuretics, with no significant between-group differences. Nitrates (32.3% vs. 24%) and inotropes (30.4% vs. 22.4%) were used in a higher proportion of the primary AHF group. Vasopressors were prescribed in 38% of patients, with no differences between the primary and secondary groups (36.7% vs. 39.3%, respectively).
Coronary angiography was performed in a higher proportion of patients in the primary group (35.1% vs. 22.7%). Mechanical circulatory support was necessary in 2.9% of patients with AHF. The use non-invasive ventilation was higher in the primary group (40.4% vs. 27.9%). Invasive mechanical ventilation was used in 33.5% of patients, with no differences between the primary and secondary groups.
Length of stay and mortalityPatient outcomes are shown in Table 3. Compared to the patients without AHF, those diagnosed with AHF had longer mean ICU (6 vs. 9 days) and hospital (19 vs. 23 days) stays. Compared to the primary group, the secondary group had a longer hospital stay (19 vs. 27 days).
Outcomes.
| Acute heart failure | AHF subgroup | |||||
|---|---|---|---|---|---|---|
| Non=3703 | Yesn=627 | p value | Primaryn=319 | Secondaryn=308 | p value* | |
| Length of stay, days | ||||||
| ICU | 6 (6–7) | 9 (8–10) | <0.001 | 9 (7–10) | 10 (9–12) | 0.198 |
| Hospital | 19 (18–20) | 23 (21–26) | <0.001 | 19 (18–22) | 27 (23–31) | 0.001 |
| Mortality | ||||||
| ICU | 308 (8.3%) | 99 (15.7%) | <0.001 | 54 (17.4%) | 45 (15.5%) | 0.530 |
| 30-day | 317 (8.6%) | 111 (17.7%) | <0.001 | 62 (19.4%) | 49 (15.9%) | 0.247 |
| Hospital | 408 (11%) | 135 (21.6%) | <0.001 | 72 (23.8%) | 63 (23.3%) | 0.886 |
AHF: acute heart failure; ICU: intensive care unit.
Data are presented as n (%) or mean (95% confidence intervals).
Patients with AHF had significantly greater risks of ICU mortality (odds ratio [OR] 2.07 [95% CI: 1.62–2.64]; p<0.001) and 30-day mortality (OR 2.45 [95% CI 1.93–3.11]; p<0.001) than patients without AHF.
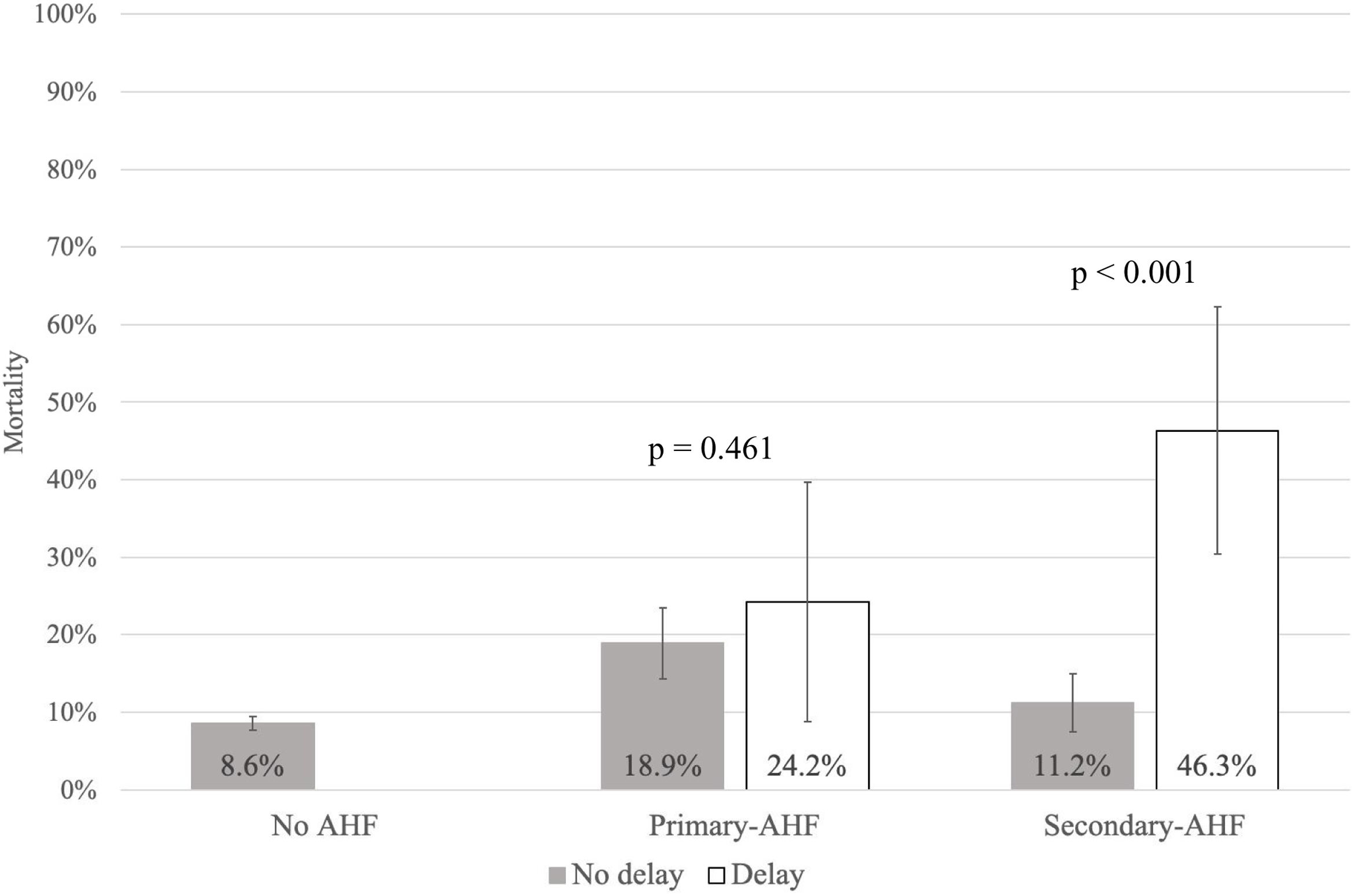
Detailed results of the univariate analysis of all risk factors associated with 30-day mortality in the AHF group are shown in Table S1. On the Cox regression analysis, adjusted for confounding factors, the following variables were independently associated with 30-day mortality: increase in APACHE II, presence of cardiogenic shock, left ventricular ejection fraction, need for early inotropic therapy, and diagnostic delay (Table 4). Comparison of the impact of diagnostic delay on 30-day mortality in the two AHF groups showed a significant effect in the secondary group (OR: 6.82; [95% CI: 3.31–14.04]; p<0.001) (Fig. 2).
Univariate and Cox regression analyses of factors associated with mortality at 30 days from ICU admission in the acute heart failure groups (n=627).
| Univariate analysis | Cox regression analysis | |||
|---|---|---|---|---|
| Variable | OR (95% CI) | p value | Adjusted hazard ratio (95% CI) | p value |
| Age (for each additional year) | 1.02 (1.001–1.0.4) | 0.03 | 1.02 (0.99–1.04) | 0.18 |
| APACHE II (for each one point increase) | 1.08 (1.05–1.10) | <0.001 | 1.04 (1.01–1.07) | 0.004 |
| Previous history ischemic heart disease, yes | 1.80 (1.17–2.70) | 0.007 | 1.79 (0.99–3.28) | 0.06 |
| Cardiogenic shock, yes | 5.94 (3.70–9.56) | <0.001 | 4.01 (1.66–9.67) | 0.002 |
| Left ventricular ejection fraction (for each percentage point increase) | 1.60 (0.95–2.71) | 0.07 | 0.97 (0.95–0.99) | 0.01 |
| Early vasopressor treatment, yes | 4.99 (3.20–7.79) | <0.001 | 1.45 (0.59–3.56) | 0.42 |
| Early inotropic treatment, yes | 2.33 (1.52–3.59) | <0.001 | 2.37 (1.14–4.94) | 0.02 |
| Early nitrate treatment, yes | 0.43 (0.25–0.74) | 0.002 | 0.41 (0.15–1.06) | 0.06 |
| Invasive mechanical ventilation, yes | 4.58 (2.97–7.06) | <0.001 | 1.39 (0.68–2.87) | 0.36 |
| Diagnostic delay, yes | 2.99 (1.77–5.08) | <0.001 | 2.39 (1.31–4.36) | 0.005 |
OR: odds ratio; CI: confidence interval; APACHE: Acute Physiology and Chronic Health Evaluation.
Impact of diagnostic delay on 30-day mortality by acute heart failure group. 30-Day mortality rate by group depending on the presence (white bars) or not (grey bars) of diagnostic delay. The bars give the mean mortality rate and the lines 95% confidence intervals. The p value indicates the comparison between patients with and without diagnostic delay in each AHF subgroup. AHF: acute heart failure.
In this multicentre, prospective cohort study, we assessed the incidence, diagnosis, and prognostic impact of AHF in critically ill patients. AHF was evaluated as the main reason for ICU admission (primary group) and as a complication during ICU stay (secondary group). The most important finding of this study is that a delay>12h in the diagnosis of AHF during the ICU stay (secondary AHF) was associated with an increased risk of 30-day mortality (OR: 6.82).
The overall incidence of AHF in this cohort was 14.5%. Unfortunately, previous studies have only evaluated the incidence of AHF in cardiac critical care units (ranging from 20% to 69%) rather than in the ICU, and thus our data are not comparable.7–10 Our study population provides a broader perspective of AHF, which may explain the lower incidence of AHF as the reason for ICU admission. Although some studies have evaluated the incidence of AHF during ICU stays, they have done so only in certain clinical scenarios such as sepsis or weaning from mechanical ventilation.18,19 To our knowledge, ours is the first study to assess the incidence of AHF in a general cohort of critically ill patients throughout their ICU stay, showing that primary and secondary AHF had a similar incidence rate in this cohort.
The patients with primary and secondary AHF differed significantly in terms of etiologic and precipitating factors. The primary group was mostly comprised of patients with pulmonary oedema caused by primary heart disease. A similar pattern of presentation was described in the French AHF study (EFICA)9 and in the international AHF study (ALARM-HF).7 By contrast, our results differed from the Romanian AHF study (RO-AHFS),10 in which cardiogenic shock was more prevalent than pulmonary oedema.
The secondary group was comprised of patients whose heart was already debilitated or acutely stressed and who developed AHF in the ICU due to infection, fluid overload, and/or weaning from mechanical ventilation. Fluid overload was the trigger for a one-quarter of these cases of AHF. Although the need to achieve positive fluid balance may be justified as part of the initial management of the underlying disease (i.e., the condition leading to ICU admission),20 our findings underscore the need for a more comprehensive and continuous analysis of fluid status21 in these patients in order to implement preventative measures or to start de-resuscitation or active fluid removal.22
The mean length of stay in the ICU and hospital in our AHF patients was higher than described in the literature.7,9,10 This finding makes sense given that the secondary AHF group—a group that has not been evaluated in previous studies—had the longest hospital stay, thereby raising the mean length of stay of the combined cohort. The hospital mortality rate in the AHF patients was similar to that reported in the ALARM-HF7 and the RO-AHFS10 studies (17.8% and 17.5%, respectively), while 30-day mortality was slightly lower than that reported in the EFICA study (43.2%).9 Importantly, the mortality rate in patients with AHF was more than double that of the patients without AHF. On the multivariable analysis, four non-modifiable factors (severity at admission, cardiogenic shock, left ventricular ejection fraction, and need for inotropic treatment) and one modifiable factor (diagnostic delay) were independently associated with mortality in AHF patients.
Diagnostic delay was present in 11.8% of the study population and associated with a significant increase in mortality in the secondary AHF group. Early detection and timely administration of appropriate treatment are the two most important measures to improve outcomes in patients with AHF.23 However, the initial signs and symptoms of AHF, especially in critically ill patients, are frequently non-specific. To avoid diagnostic delay in these patients, we can use non-invasive tools such as echocardiography or determination of natriuretic peptides concentrations.
B-type natriuretic peptides and NT-proBNP have both proven useful to rule out the presence of heart failure in critically ill patients. Serum concentration levels of these peptides have been correlated with left ventricular filling pressure,24 even in patients who develop AHF during ICU stay.25–27 However, natriuretic peptide levels were determined in only 34.6% of our patients, most likely because these levels are non- specific in critically ill patients in whom many non-cardiac factors (e.g., older age, female sex, renal dysfunction) can alter peptide concentrations, leading to the misinterpretation of non-serial peptide measurements.28
Echocardiography was performed during the ICU stay in approximately three-quarters (73.4%) of the patients with AHF. Although this percentage may seem low, it is consistent with the proportion of patients with a history of heart failure and previously known cardiac function in the AHF group (33.4%). Guidelines in place during the study period4 recommend immediate echocardiography only in patients with hemodynamic instability or with suspicion of acute life-threatening abnormalities, and early echocardiography in patients with de novo AHF or unknown cardiac function. Left ventricular function was preserved in 40% of our patients; however, data on diastolic function were available in only a low proportion of patients. While systolic function can be qualitatively assessed from the visual ejection fraction, there is no such simple index to assess diastolic function, which requires more complex and time-consuming echocardiographic methods.15 In this regard, determination of left ventricular diastolic function should be considered in ICU patients due to the essential role it plays in ICU-acquired AHF27,29,30 and because it can help identify the presence of left ventricular overload to guide decongestive therapy.31
Strengths and limitationsThis study has several limitations. The first limitation is related to the pragmatic real-life/bedside study design in which diagnosis was based on the treating physician's clinical criteria (based on signs, symptoms, and complementary tests), with a focus on analysing clinical outcomes in routine clinical practice. Second, due to the observational nature of the study, it is important to underscore that our results only indicate an association rather than causality. Third, there may be a selection bias in the patients admitted to the ICU for AHF given the potential for differences in ICU admission criteria among the 32 participating hospitals. Fourth, diagnostic delay was registered as a dichotomous variable (present or not) and thus we do not know if longer delays are associated with worse prognosis, a question that should be explored in future studies. Finally, this study was conducted in Spain and our findings might not be applicable in other countries with different health care systems and policies.
The main strengths of our study include the prospective multicentre real-life design and the study population. This study design allowed us to analyse routine clinical practice and to identify areas of improvement in the future management of AHF patients. Finally, the selection of this specific patient population—a general cohort of critically ill patients observed throughout their ICU stay—has allowed us to identify a subpopulation (secondary AHF) not present in previous studies.
ConclusionsAHF is common in critically ill patients, either as the main admitting diagnosis or as a complication of other critical illnesses. Patients who develop AHF during ICU admission have an increased risk mortality when diagnosis is delayed. To minimise the risk of heart failure in ICU patients, it is essential to avoid modifiable precipitating factors, particularly fluid overload through appropriate preload monitoring, and to ensure early diagnosis in order to initiate treatment as soon as possible.
Authors’ contributionsLZ, CG and RG designed the study, performed data collection, analysed the data and wrote the manuscript. FRC participated in data collection, analysed the data and wrote the manuscript. TGP, LC, EP, IR, IL, AFF, IAH performed data collection and review the final version of the manuscript. The ICA-UCI study group performed data collection. All authors read and approved the final manuscript.
Ethics approval and consent to participateThe study protocol was approved by the ethics committee of each participating hospital.
FundingNo funding was received for this work and publication.
Conflict of interestsThe authors declare that they have no competing interests.
ICA-UCI study group: María José Chaparro-Sánchez (Hospital Regional Universitario de Málaga. Málaga), Míriam Lafuente-Mateo (Hospital Nuestra Señora de Gracia. Zaragoza), Concepción Tarancón-Maján (Hospital Virgen de la Concha. Zamora), Gerardo Ferrigno-Bonilla (Hospital Virgen de la Concha. Zamora), Samuel Fernández-Vilches (Consorci Corporació Sanitària Parc Taulí. Sabadell. Barcelona), Gemma Gomà-Fernández (Consorci Corporació Sanitària Parc Taulí. Sabadell. Barcelona), Diego Pablo Rodríguez-Giardinieri (Consorci Sanitari de Terrassa. Terrassa. Barcelona), Christian Villavicencio-Luján (Hospital Universitario Joan XXIII. Tarragona), Marina Garcia-de-Acilu (Hospital Universitari Vall d’Hebron. Barcelona), Olga Moreno-Fontanillas (Hospital Universitari General de Catalunya. Sant Cugat del Vallès. Barcelona), María Luisa Martínez-González. (Hospital Universitari General de Catalunya. Sant Cugat del Vallès. Barcelona), Elisabeth Navas-Moya (Hospital Universitario Mútua Terrassa. Terrassa. Barcelona), Xavier Esquirol-Puig (Hospital General de Granollers. Granollers. Barcelona), Javier Ruiz-Ruiz (Hospital de Llíria. Llíria. Valencia), Enver Rodríguez-Martínez (Hospital General de Castellón. Castellón), Alicia Barrios-Pérez (Hospital Universitario de La Ribera. Alzira. Valencia), Lucia Arias-Portaceli (Hospital Universitario de La Ribera. Alzira. Valencia), Ana Abalos-García (Hospital Universitario de La Ribera. Alzira. Valencia), Ana María Mancilla-Arias (Hospital de Mérida. Mérida. Badajoz), Miguel Antonio Solla-Buceta (Complexo Hospitalario Universitario A Coruña. A Coruña), Leticia Seoane-Quiroga (Complexo Hospitalario Universitario A Coruña. A Coruña), María Teresa Bouza-Vieiro (Complexo Hospitalario Universitario A Coruña. A Coruña), María José de la Torre-Fernández (Complexo Hospitalario Universitario de Ourense. Ourense), Francisco Javier Cid-López (Complexo Hospitalario Universitario de Ourense. Ourense), Carmen Josefina Fernández-González (Complexo Hospitalario Universitario de Ferrol. Ferrol. A Coruña), Beatriz Besteiro-Grandío (Clínica Juaneda Menorca. Ciutadella. Menorca), Raúl Vicho-Pereira (Hospital Quirónsalud Palmaplanas. Palma de Mallorca. Mallorca), Judith Cabrera-Rivero (Hospital Universitario de Canarias. Santa Cruz de Tenerife), Andrea Carolina Álvarez-Castillo (Hospital Universitario de Canarias. Santa Cruz de Tenerife), Inés Torrejón-Pérez (Hospital del Henares. Alcalá de Henares. Madrid), Marcela Hómez-Guzmán (Hospital del Henares. Alcalá de Henares. Madrid), Sara Helena de Miguel-Martín (Hospital General de Villalba. Collado Villalba. Madrid), José Higinio de Gea-García (Hospital Clínico Universitario Virgen de la Arrixaca. Murcia), Silvia Sánchez-Cámara (Hospital Universitario Virgen de la Arrixaca. Murcia), Juana María Serrano-Navarro (Hospital General Universitario Reina Sofía. Murcia), Ivan Keituqwa-Yáñez (Hospital Rafael Méndez. Lorca. Murcia).
We would like to thank the invaluable help of the Epidemiology Department of Hospital de la Santa Creu i Sant in the development and analysis of this study.
We thank Bradley Londres, biomedical editor, for reviewing and editing de manuscript.