As calculated by the severity scores, an unknown number of patients are admitted to the Intensive Care Unit (ICU) with a very high risk of death. Clinical studies have poorly addressed this population, and their prognosis is largely unknown.
DesignPost hoc analysis of a multicenter, cohort, longitudinal, observational, retrospective study (CIMbA).
SettingSixteen Portuguese multipurpose ICUs.
PatientsPatients with a Simplified Acute Physiology Score II (SAPS II) predicted hospital mortality above 80% on admission to the ICU (high-risk group); A comparison with the remaining patients was obtained.
InterventionsNone.
Main Variables of InterestHospital, 30 days, 1 year mortality.
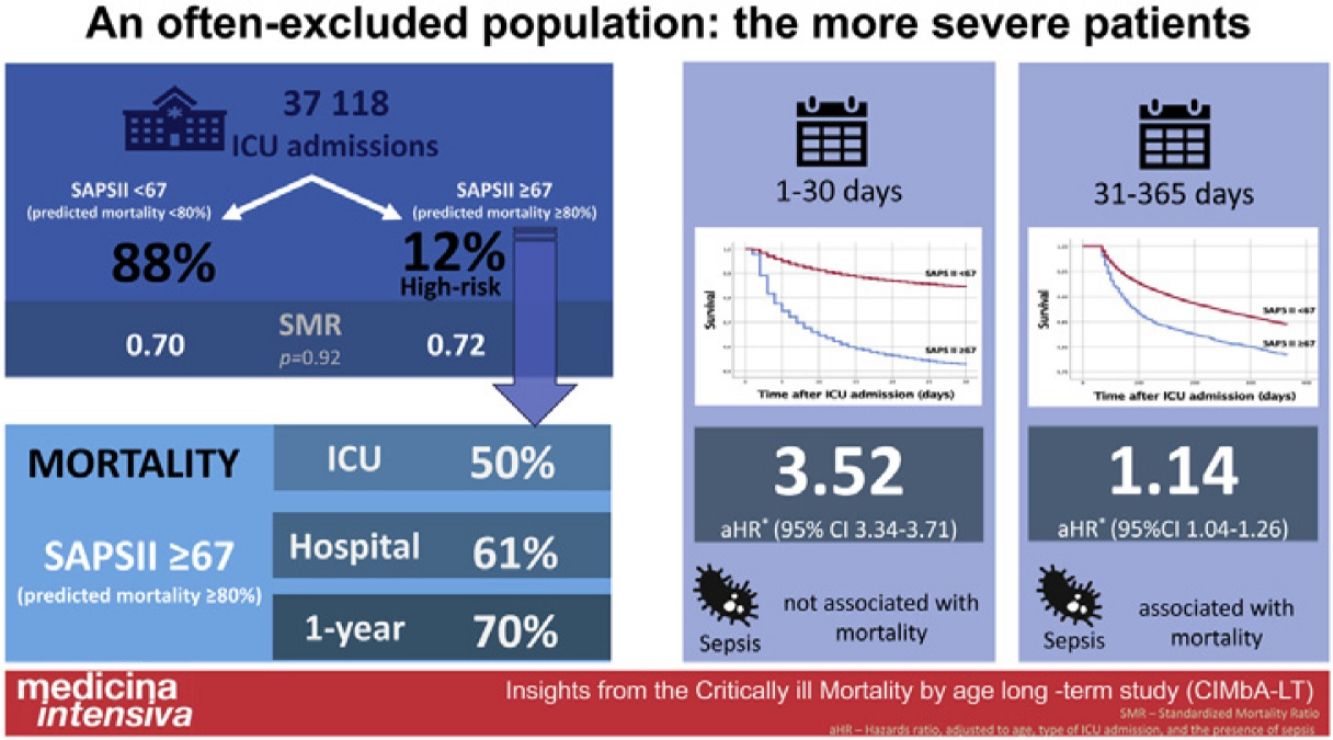
ResultsWe identified 4546 patients (59.9% male), 12.2% of the whole population. Their SAPS II predicted hospital mortality was 89.0±5.8%, whilst the observed mortality was lower, 61.0%. This group had higher mortality, both during the first 30 days (aHR 3.52 [95% CI 3.34–3.71]) and from day 31 to day 365 after ICU admission (aHR 1.14 [95%CI 1.04–1.26]), respectively. However, their hospital standardized mortality ratio was similar to the other patients (0.69 vs. 0.69, P=.92). At one year of follow-up, 30% of patients in the high-risk group were alive.
ConclusionsRoughly 12% of patients admitted to the ICU for more than 24h had a SAPS II score predicted mortality above 80%. Their hospital standardized mortality was similar to the less severe population and 30% were alive after one year of follow-up.
Según las escalas de gravedad, un número indeterminado de pacientes ingresan en la Unidad de Cuidados Intensivos (UCI) con riesgo de muerte muy elevado. Este grupo ha sido poco abordado en los estudios clínicos y se desconoce en gran medida su pronóstico.
DiseñoAnálisis post-hoc de estudio multicéntrico, de cohortes, longitudinal, observacional y retrospectivo (CIMbA).
ÂmbitoDieciséis UCI polivalentes portuguesas.
PacientesPacientes con mortalidad hospitalaria prevista en el Simplified Acute Physiology Score II (SAPS II) superior al 80% nel ingreso en la UCI (grupo de alto riesgo); se compararon con los restantes.
IntervencionesNinguna.
Variables de interés principalsMortalidad hospitalaria, a 30 días y 1 año.
ResultadosSe identificaron 4546 pacientes (59.9% hombres), 12.2% da población. La mortalidad hospitalaria estimada por lo SAPS II fue de 89.0±5.8%, aunque la observada fue inferior, 61.0%. Este grupo presentó mayor mortalidad, tanto durante los primeros 30 días (aHR 3.52 [IC 95%: 3.34–3.71]) y desde el día 31 hasta el día 365 después del ingreso en UCI (aHR 1.14 [IC 95%: 1.04–1.26]). Sin embargo, su índice de mortalidad hospitalaria estandarizada fue similar a los otros pacientes (0.69 vs. 0.69; P=.92). Al primer año de seguimiento, 30% de los pacientes de alto riesgo estaban vivos.
ConclusionesAproximadamente 12% de los pacientes ingresados en la UCI durante más de 24 horas tenían una mortalidad prevista por SAPS II superior al 80%. Su mortalidad hospitalaria estandarizada fue similar a la de la población menos grave y el 30% estaban vivos después de un año de seguimiento.
Critical care medicine is designed for patients with critical illnesses at different stages of their condition. There is an identifiable ongoing morbidity and mortality attributable to the acute severe illness event, to older age, and to a growing number of comorbidities.
All-cause mortality is a common outcome measure in the Intensive Care Unit (ICU), used as a major primary endpoint in several cohort studies and benchmarking analyses, and as a surrogate of the quality of care.1 Scoring systems, like the commonly used Simplified Acute Physiology Score (SAPS) II,2 help to describe ICU populations and interpret the outcome measures between different populations and ICUs.3–6
A growing number of patients have a very severe disease on ICU admission and a very high risk of death, as predicted by the severity scores, due both to older age and age-related syndromes, especially comorbidities.7
This most severely ill population is poorly studied in clinical studies, due to predicted worst outcomes, and their prognosis is largely unknown.8,9
A better knowledge of their ICU and Hospital outcomes, along with the identification of risk factors for short- and long-term mortality is warranted.
This study's goal was to assess short-term (hospital and 30-day) and long-term (one and two years) outcomes of critically ill patients with SAPS II predicted mortality above 80% admitted to ICU in Portugal. We intend to quantify their mortality risk at different time points to support prognostication and to help patients (or their relatives) and doctors to inform realistic goals of care.
Patients and methodsThis is a post hoc analysis of Critically ill Mortality by age long-term (CIMbA LT) study, a multicenter, cohort, longitudinal, observational, retrospective study, that included 16 different Portuguese intensive care departments. The study protocol has been published elsewhere.10 Briefly, data from all adult patients admitted to one of the 16 participating ICUs, for more than 24h, between January 1, 2015, and June 10, 2019, were included. Only the first ICU admission of any patient was considered. Demographic, clinical, and outcome data, along with the SAPS II score, were collected. The SAPS II score is largely used in Portugal including for ICU benchmarking and locally measured data was included in the study. Nevertheless, all participating ICUs were specifically recommended to confirm all unplausible, or missing data. A final check was performed on the whole data, plotting SAPS II against hospital mortality, to find potential incongruencies. The one and two-year follow-up was accomplished through direct patient or relatives contact, hospital registries, or from the National Health Directory database. At least, one year of follow-up was completed for each patient.
Anonymized data were introduced in a file created specifically for this study and all patients were identified by a consecutive number. Missing data on age or SAPS-II score led to database exclusion.
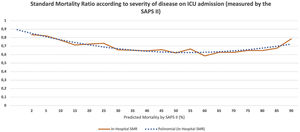
We calculated the standardized mortality rate (SMR, that is the ratio between observed and predicted hospital mortality according to the SAPS II score) for patients admitted with each SAPS II score, both at hospital discharge and at one year of follow-up. We plot the results to evaluate if there was a cut-off point (Fig. 1).
Standard mortality ratio according to severity of disease on Intensive care Unit admission (measured by the SAPS II), calculated at hospital discharge. The non-continuous line represents polynomial function for the SMR dispersion trend (y=0,0019x2−0,0494x+0,941). ICU, Intensive Care Unit; SAPS, Simplified Acute Physiology Score; SMR, Standardized Mortality Ratio.
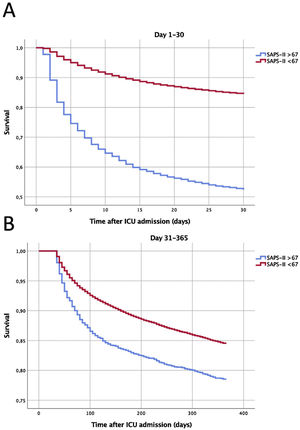
Accordingly, we arbitrarily defined the most severe group as those with a SAPS II score≥ 67 (corresponding to predicted hospital mortality of 80%). These were segregated for further analysis. Their first 30 days' cumulative ICU mortality was mapped, to envisage the days with higher mortality (Fig. 2).
Survival curves according to the severity of disease on the Intensive Care Unit admission. Panel A: Survival during the first 30 days after admission. Panel B: Long-term cumulative survival in 30-day survivors (from the 31st to the 365th day of follow-up). Log Rank test P<.001 for both. SAPS, Simplified Acute Physiology Score; ICU, Intensive Care Unit.
We calculated the ICU and the Hospital length of stay (LOS) and the all-cause mortality for the group with higher SAPS II predicted mortality and the remaining patients. Survival curves for both groups were plotted separately, for both the whole population and according to the presence of sepsis on admission to the ICU. Sub-analyses of the first 30 days and for the 31st to 365th days after ICU admission were also obtained. The relationship between age and mortality during these two time periods was calculated.
The study was approved by the local Research and Ethics Committees of all the participating centers. According to the study design, informed consent was waived.
Statistical analysisDescriptive statistics were calculated. Data were summarized as mean±standard deviation or median [percentile 25 and 75, p25–p75], according to data distribution. Categorical variables were described as N (%).
The chi-square test was used to compare categorical variables, whilst continuous variables were evaluated with the Student T test or the Mann-Whitney U test, according to data distribution.
The Cox proportional hazard was used to compare the group with higher SAPS II predicted mortality with the remaining patients. The hazards ratio, adjusted to age, type of ICU admission, and the presence of sepsis (aHR), with the respective 95% confidence interval (CI) were computed, both for the first 30 days after ICU admission and, for the 30-day survivors, for the 31st–365th days of follow-up. The log-rank test was calculated for comparisons between sub-groups in patients with or without sepsis. The impact of age on mortality at each of the defined time points was calculated with the Chi-square test.
Statistical analysis was performed using IBM SPSS Statistics v.25.0 (IBM, Somers, NY, USA). All statistics were 2-tailed and the significance level was defined as P<.05.
ResultsWe evaluated 37,118 patients, of whom 4546 (12.2%) had a SAPS II≥67 (the high-risk group). Their demographic characteristics are presented in Table 1. More than 70% were older than 65 years (compared with 50.0% of the remaining population, P<.001) and 61% were male (vs. 59.9%, P=.17). A medical reason for admission and the presence of sepsis were both significantly more common in the high-risk group (Table 1 and Supp Table 3).
Demographic characteristics.
| SAPS≥67 (N 4546) | SAPS<67 (N 32,572) | P-value | ||
|---|---|---|---|---|
| Age(years) | 18−50 | 341 (7.5%) | 7291 (22.4%) | <.001 |
| 51−65 | 935 (20.4%) | 8998 (27.6%) | ||
| 66−80 | 2205 (48.5%) | 11,462 (35.2%) | ||
| >80 | 1065 (23.4%) | 4821 (14.8%) | ||
| Gender | Male | 2773 (61%) | 19,521 (59.9%) | .17 |
| Female | 1773 (39%) | 13,051 (40.1%) | ||
| Type of admission | Medical | 3197 (70.3%) | 20,173 (61.9%) | <.001 |
| Unscheduled Surgery | 1244 (27.4%) | 8115 (24.9%) | ||
| Scheduled Surgery | 105 (2.3%) | 4284 (13.2%) | ||
| Sepsis | 1971 (43.4%) | 9477 (29.1%) | <.001 | |
| ICU LOS (days) (median, p25–p75) | 5.4 [1−27] | 4 [1−24] | <.001 | |
| Hospital LOS (days) (median, p25–p75) | 9 [2−72] | 13 [1−67] | <.001 | |
SAPS II, Simplified Acute Physiology Score II; ICU, Intensive Care Unit; LOS, Length of Stay; p25–p75, Percentiles 25 and 75.
More than half of the deaths (51.1%) during the first month after ICU admission occurred during the first 4 days (Fig. 2, panel A).
The predicted hospital mortality (according to the SAPS II score) in this group was very high, 89.0±5.8%. The observed hospital mortality was lower, 61.1%, with an SMR of 0.69, and this was similar to the SMR of the less severe population (Fig. 1 and Table 2).
Patients’ mortality at the different time points after Intensive Care Unit admission.
| SAPS≥67 | SAPS<67 | P-valuea | |
|---|---|---|---|
| SAPS II predicted hospital mortality | 89.0±5.8% | 27.4±22.8% | |
| ICU Mortality | 2250 (49.5%) | 3731 (11.5%) | <.001 |
| Hospital Mortality | 2775 (61.0%) | 6141 (18.9%) | <.001 |
| SMR | 0.69 | 0.69 | .92 |
| 1-year mortality | 3184 (70.0%) | 9931 (30.5%) | <.001 |
SAPS II, Simplified Acute Physiology Score II; ICU, Intensive Care Unit; SMR, Standardized mortality ratio.
The aHR for 30-day all-cause mortality for this high-risk population was 3.52 (95% CI 3.34–3.71). When addressing only the 30-day survivors, the mortality risk remained significantly higher during the first year of follow-up, days 31st–365th aHR 1.14 (95%CI 1.04–1.26) (Fig. 2, panel B).
After the first year of follow-up, 70.0% of the population with a SAPS II predicted hospital mortality above 80% had died (Table 2), a figure which remained, however, well below the SAPS II initial predicted mortality.
This group had slightly longer ICU LOS but shorter Hospital LOS (Table 1). However, this difference was related to early mortality. When addressing only survivors, this high-risk group has longer ICU and hospital LOS (8 [1.9–31.4] vs 4 [1–23.7], P<.001 and 21.5 [5.0–96.6] vs. 14 [3–73], P<.001, respectively).
Of the whole population, 30.8% of patients were admitted to the ICU with sepsis, and this diagnosis was associated with worst outcomes (Suppl Table 3). However, this difference was much more striking in the less severe, group (odds ratio 1.78, 95% CI 1.68–1.89), than in the population with the higher SAPS II predicted mortality (odds ratio 1.12, 95%CI 0.99–1.27). Curiously, the mortality rate during the first 30 days was also not influenced by age in this high-risk group (P=.924), as opposed to what was noted during the late period and in the less severe patients (Supp Figs. 3 and 4).
DiscussionIn this study, we evaluated the 12.2% of the most severely critically ill patients admitted to the participating ICUs, with a SAPS II predicted hospital mortality of 89.0±5.8%. Their hospital mortality, albeit lower, was still 61.0%. More than half of the first-month deaths of this group occurred during the first 4 days of ICU stay. Despite this high, and early, mortality rate, their hospital standardized mortality ratio was 0.69, in line with what was found in the remaining, less severe patients. Despite their high severity at ICU admission, at one year of follow-up, roughly 30% of this population were still alive.
Different risk factors for early and late mortality of critically ill patients have been identified. Those often include sepsis and infection (either on admission or complicating the course of stay), the severity of illness at ICU admission (measured by different scores), age, the presence of comorbidities, functional status at ICU admission, and the need for ICU readmission. All these were significantly associated with both early (30 days) and late (31–365 days) mortality in a recently published observational study,11 results which were similar to ours. Interestingly, in our population, in patients with high SAPS II predicted mortality, sepsis only impacted late mortality (Supp Table 3 and Fig. 4).
Differences in ICU outcomes may be related to the available resources, as recently shown by Martin-Loeches et al.12 In their study, late deaths were associated with older age and infection. Significantly, patients from higher-income countries have a higher rate of late deaths, probably related to the higher availability of ICU beds that allow treatment of a more chronically diseased and frail group of patients. Portugal is a middle-income country with a relatively short number of ICU beds (increasing from 4.2 beds per 100,000 inhabitants in 201213 to 6.4 at the end of the study period14), and this may have influenced our results. The lack of availability of ICU beds is associated with a worse prognosis, even when a delayed admission is possible.15 That calls for action aiming at judicious resource use and providing the optimal intensity of care according to patients' potential benefit.
Roughly 30% of our high-risk patients were alive after one year, unveiling a lasting benefit of their ICU admission. Pintado et al. evaluated 88 patients who were refused ICU admission because they were “too ill to benefit”. Those patients had more comorbidities and worse mental and functional outcomes but roughly one quarter were alive after one year,16 suggesting that prognostication during the acute disease may be problematic,17 even in more severe patients. In fact, our study showed an SMR lower than one, translating into an improved prognosis compared to SAPS II predicted hospital mortality. This benefit was similar in the whole spectrum of disease severity, with no obvious cut-off point. Even patients older than 75 years may have a long-term (one year) benefit of ICU admission, although prolonged ICU stay may jeopardize this benefit, leading to lower survival and poor quality of life.18
Consequently, an ICU trial,19 that is, admitting and treating the patient for a predefined short period, followed by withdrawal if no benefit is found, may help to surpass this dilemma. This may also help to reduce the provision of excessive critical care resources to patients who appropriately enter the ICU.20 It may also facilitate an early definition of realistic goals of care, which may improve patients’ and families’ satisfaction and contribute to better use of resources.21,22
Andersen et al.23 focused on advanced age as an independent predictor of ICU mortality. In his study, most of the ICU deaths occurred very early, during the first 2 days after admission, mostly related to life-supporting withdrawal. In our cohort, more than half of the deaths of the severest patients happened during the first four days after admission (Fig. 2). Of note, we excluded patients who died during the first 24h in the ICU, and the high-risk group was selected based on severity (assessed by SAPS II score) and not only by age. Consequently, an early withdrawal decision was probably less likely to happen.
Our data seems to suggest that even very severe patients, who survive after the fourth day, do not necessarily have a dismal prognosis, and may experience long-term survival. Accordingly, we think that no withdrawal decisions should be based only on clinical severity during the ICU stay.
Clinical severity, age, comorbidities, and length of ICU stay have also been related to post-ICU mortality.24 Similarly, in our population, there were more late deaths in our high-risk group (aHR 1.14, 95%CI 1.04–1.26), even after being discharged alive from the hospital.
These observations may have important organizational implications and challenge admission policies. Future research should focus on the improvement of patient-centered prognostic scores, including individual characteristics (such as frailty, and comorbidities), assessing not only hospital outcomes but also short- and long-term morbidity and mortality, and facilitating patients' own choices. The development and evaluation of interventions aiming to improve the long-term outcome of high-risk patients admitted to the ICU should also be a priority.
We acknowledge that our study has several limitations. First, our sample was limited to a group of ICUs in a single country, and the short and long-term outcomes of severe patients admitted to ICU may differ across countries. Second, our database does not include information on functional status before admission, although severely dependent patients are usually excluded from admission.17 Third, we excluded patients with less than 24h of ICU stay, including those who died. Fourth, the SAPS II score may fail to capture all the severity of patients, including their frailty, that may influence the outcomes,25 and we were not able to formally check the local accuracy of the SAPS II calculation. Fifth, we did not include other severity scores. Finally, we did not collect data on the level of life-support, treatment limitations, end-of-life decisions, the policy of ICU trials,19 and causes of death after ICU discharge.1
Our work had also some strengths: it included a large ICU database and focused on a generally poorly studied sub-group. To our knowledge, this is the first study to analyze a large database of the long-term outcome of a population with a very high predicted risk of death admitted to an ICU.
ConclusionMore than 12% of patients admitted to the ICU had very high predicted hospital mortality according to SAPS II. These patients often died during the first 4 days after ICU admission and had a short- but also long-term increase in the risk of death. Nevertheless, roughly 30% were alive after one year of follow-up.
FundingNone.
Conflict of interestNone.
Contribution of the authorsAO, JGP designed the study; AO, TV, ARR, NJ, LT, and LC acquired the data and performed a literature search; AO, JGP check the data for missing or implausible values; AO, JGP, TV, JAP analyze and interpret the data; JGP, AO, JAP drafted the manuscript; AO, JGP; TV; NJ, LT, LC, JAP revised the manuscript for important intellectual content; JGP, AO provided the statistical expertise. JGP acts as the guarantor of the integrity and accuracy of the data. All authors read and approved the final manuscript.
Collaborators of the CIMBA-LT study:
Hospital Vila Franca de Xira: André Oliveira; João Gonçalves-Pereira; Joaquim Lima. Centro Hospitalar de Médio Tejo (Abrantes): Rui Assis; Joana Monteiro. Hospital Nélio Mendonça (Funchal): André Simões; Catarina Lume. Centro Hospitalar de Trás-os-Montes e Alto Douro (Vila Real): Maria João Pinto. Centro Hospitalar de Vila Nova de Gaia: Sara Pipa. Hospital de Braga: Laura Costa. Hospital de Bragança: Cristina Nunes. Hospital do Divino Espírito Santo (S. Miguel): Manuela Henriques; Luís Tavares. Hospital de Leiria: Filipa Sequeira. Centro Hospitalar Universitário de S. João (Porto): José-Artur Paiva; Tatiana Santos Vieira; Núria Jorge. Centro Hospital Universitário de Lisboa Norte (Lisboa): Ana Bento Rodrigues; Susana Fernandes; João Ribeiro. Hospital S. Francisco Xavier (Lisboa): Rui Morais; Pedro Póvoa; Luís Coelho. Centro Hospitalar Universitário de Coimbra: Ana Martinho; Iolanda Santos. Hospital Egas Moniz (Lisboa): Gabriela Almeida. Hospital de Beja: Alexandra Paula; Filipe Morais de Almeida. Centro Hospitalar Universitário do Algarve (Faro): Sofia Ribeiro.