Editado por: Federico Gordo - Medicina Intensiva del Hospital Universitario del Henares (Coslada-Madrid)
Última actualización: Octubre 2023
Más datosTo assess the impact of a multimodal interventional project (“Zero Resistance”) on the acquisition of multidrug-resistant bacteria (MDR-B) during the patient’s ICU stay.
DesignProspective, open-label, interventional, multicenter study.
Setting103 ICUs.
PatientsCritically ill patients admitted to the ICUs over a 27-month period.
InterventionsImplementation of a bundle of 10 recommendations to prevent emergence and spread of MDR-B in the ICU.
Main variable of interestRate of patients acquiring MDR-B during their ICU stay, with differentiation between colonization and infection.
ResultsA total of 139,505 patients were included. In 5409 (3.9%) patients, 6020 MDR-B on ICU admission were identified, and in 3648 (2.6%) patients, 4269 new MDR-B during ICU stay were isolated. The rate of patients with MDR-B detected on admission increased significantly (IRR 1.43, 95% CI 1.31–1.56) (p<0.001) during the study period, with an increase of 32.2% between the initial and final monthly rates. On the contrary, the rate of patients with MDR-B during ICU stay decreased non-significantly (IRR 0.93, 95% CI 0.83–1.03) (p=0.174), with a 24.9% decrease between initial and final monthly rates. According to the classification into colonization or infection, there was a highly significant increase of MDR-B colonizations detected on admission (IRR 1.69, 95% CI 1.52–1.83; p<0.0001) and a very significant decrease of MDR-B-infections during ICU stay (IRR 0.67, 95% CI 0.57–0.80, p<0.0001).
ConclusionsThe implementation of ZR project-recommendations was associated with a significantly reduction an infection produced by MDR-B acquired during the patient’s ICU stay.
Evaluar el impacto de un proyecto de intervención multimodal (“Resistencia Zero”, RZ) en la adquisición de bacterias multirresistentes (BMR) durante la estancia en UCI.
DiseñoEstudio prospectivo, abierto, intervencionista, multicéntrico.
Ámbito103 UCI.
PacientesPacientes críticos ingresados en UCI, durante un período de 27 meses.
IntervencionesImplementación de un paquete de 10 recomendaciones para prevenir la aparición y propagación de BMR en UCI.
Principal variable de interésTasa de pacientes que adquieren BMR durante su estancia en UCI, diferenciando entre colonización e infección.
ResultadosSe incluyeron 139.505 pacientes. En 5.409(3,9%), se identificaron 6.020 BMR al ingreso y en 3.648(2,6%), se aislaron 4.269 nuevas BMR durante la estancia en UCI. La tasa de pacientes con BMR detectadas al ingreso aumentó significativamente (IRR 1,43, IC 95% 1,31–1,56) (p<0,001) durante el periodo de estudio, con un incremento del 32,2% entre las tasas mensuales inicial y final. Por el contrario, la tasa de pacientes con BMR detectadas durante la estancia en UCI disminuyó, no significativamente (IRR 0,93, IC 95% 0,83–1,03) (p=0,174), con una disminución del 24,9% entre las tasas mensuales iniciales y finales. Según la clasificación en colonización o infección, hubo un aumento significativo de colonizaciones por BMR detectadas al ingreso (IR 1,69, IC 95% 1,52–1,83; p<0,0001) y una disminución significativa de infecciones producidas por BMR adquiridas durante la estancia en UCI (IR 0,67, IC 95% 0,57–0,80, p<0,0001).
ConclusionesLa implementación de las recomendaciones del proyecto RZ se asoció con una reducción significativa de pacientes con infecciones por BMR adquiridas en UCI.
Antimicrobial resistance is a global threat to public health, increasing health care costs, treatment failures, and mortality.1 The critically ill patient admitted to the ICU has a greater vulnerability to infections and is particularly susceptible to be colonized/infected by multidrug-resistant bacteria (MDR-B).2,3 In Spain, infections related to invasive devices in critically ill patients have been collected for years through the National Nosocomial Surveillance Study (ENVIN-HELICS), allowing assessment of changing trends, etiology of individual infections controlled by the surveillance program, and susceptibility profile of the most frequent pathogens.4 ICU infections caused by MDR-B have similar clinical manifestations than those caused by susceptible bacteria, but treatment options are much more limited. Moreover, MDR-B infections are associated with inappropriate empirical antibiotic treatment that delays the onset of appropriate therapy, and therapeutic failure. As a consequence, mean ICU stay is prolonged, with an increase in costs, morbidity, and mortality.5,6
International organizations and scientific societies have developed strategies to reduce the emergence and spread of antimicrobial resistance, emphasizing on the need for the implementation of control measures.7–11 In Spain, the Spanish Society of Intensive Care Medicine and Coronary Units (SEMICYUC) and the Spanish Society of Intensive Nursing and Coronary Units (SEEIUC) have led two projects promoted by the Spanish Ministry of Health, Social Policy and Equality (MSSSI) aimed to reduce central venous catheter-related infections (“Bacteremia Zero” project)12 and ventilator-associated pneumonia (“Zero-VAP” project)13 in the ICU setting. The implementation of these projects has been associated with a 50% decrease of these infections and contributed to reduce antimicrobial consumption for the treatment of ICU-acquired infections. Using the structure already developed for these two projects, a new intervention project, called “Zero Resistance” was designed and implemented. The primary objective of the “Zero Resistance” project was to reduce acquisition of one or more MDR-B during the patients’ ICU stay, based on the hypothesis that a multimodal intervention or a set of specific measures applied simultaneous in daily practice can decrease the rate of colonization and/or infection by MDR-B in ICU patients. The project was approved by the Ethics Committee of ‘Parc de Salut Mar’ of Barcelona.
Materials and methodsDesign and settingA prospective, interventional, multimodal, and multicenter study was conducted in the ICU setting. ICUs for adult patients from 15 of the total 17 autonomous communities in the country were invited to participate voluntarily in the “Zero Resistance” project. The primary objective of the study was to assess the impact of a set of recommendations to prevent the development of MDR-B in critically ill patients admitted to ICUs for more than 24h. These recommendations were implemented over a 27-month period, between April 1, 2014 and June 30, 2016.
PatientsPatients admitted to Spanish ICUs were included in the study provided that the following criteria were fulfilled: a) participation in the annual Spanish registry of invasive device-associated infections in critically ill patients (ENVIN-HELICS database) between 2014 and 2016; b) provision of indicators of the “Zero Resistant” project on a monthly basis for at least 20 months during the 27 months of the study period; c) active search of MDR-B by means of surveillance cultures on ICU admission and/or during the patient’s ICU stay; and d) to complete a structural survey at the end of the implementation of the project to assess adherence to each of the bundle recommendations. Patients were prospectively and continuously followed on admission and up to 48h after discharge from the ICU.
At ICU admission, patients were classified as MDR-B carriers when MDR bacteria were isolated from surveillance cultures and/or clinical samples obtained before ICU admission or within the first 48h after admission to the ICU. Intra-ICU-acquired MDR-B were considered when an MDR bacteria, that were not present on ICU admission, were identified during the ICU stay (from 48h after admission to the ICU to 48h after transfer to another ward). Infected patients were those in which MDR-B were isolated from blood or clinical samples collected from vital organs with signs of infection, whereas colonized patients were those in which MDR-B were isolated from samples collected systematically, in the absence of signs or symptoms of infection. In the event that in the same patient with signs of infection, a BMR had been isolated in blood samples or from organs with signs of infection and, in addition, other BMR in organs or mucous membranes without signs of infection, the patients were classified as “BMR infection”. Based on the bacteria considered most problematic in Spanish ICUs, “Zero Resistance” collects information on episodes of infection and colonization of the bacteria listed in Table 1.
Definition of multidrug-resistant bacteria (MDR-B) monitored in the “Zero Resistance” program.
| Microorganism | Resistance marker |
|---|---|
| Staphylococcus aureus | Methicillin (MRSA) |
| Enterococcus spp. | Vancomycin (VRE) |
| Enterobacteriaceae | Extended-spectrum beta-lactamase (ESBL) |
| Gram negative | Carbapenems |
| Pseudomonas aeruginosa | ≥3 antipseudomonal antibiotic classes |
| Acinetobacter baumannii | Imipenem |
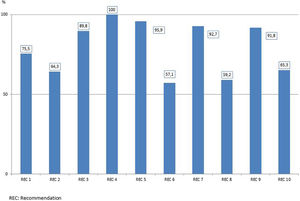
The bundle of the “Resistance Zero” project included a set of 10 specific recommendations to prevent the emergence and/or spread of MDR bacteria (Table 2), the details and rationale of which have been previously reported.14 Recommendations were selected by their potential to act on the causes that contribute to development MDR-B in hospitalized patients, namely: 1) consumption of antibiotics; 2) admission of MDR-B carriers; 3) spread of MDR-B through cross-transmission; and 4) presence of MDR-B reservoirs. Recommendations were accompanied by adherence to an integral patient safety plan, developed in the previous “Bacteremia Zero” and “VAP Zero” projects12,13 aimed to promote and enhance safety culture in ICU daily practice. In each ICU, a team of at least one intensivist and a nurse were designated as responsible for promoting and facilitating implementation of the project. Identities of the teams were communicated and registered by the healthcare authorities of each autonomous community. At the end of the implementation period, healthcare professionals completed a survey to assess adherence to each recommendation.
Recommendations of the “Zero Resistance” program to reduce patients with multidrug-resistant bacteria (MDR-B) throughout ICU stay.
| 1. In each ICU, at least one intensivist will be designated as responsible for the use of Antimicrobials. |
| 2. Empirically administer antimicrobials active against MDR-B only in cases of severe sepsis or septic shock and high risk of MDR-B based on patient risk factors and/or knowledge of local ecology. |
| 3. In each Unit, at least one nurse will be designated as leader of the “Zero Resistance” project and responsible for infection control measures aimed at reducing transmission of MDR-B. |
| 4. To perform an active search for MDR-B in all patients on admission to the unit and at least once a week throughout their stay. |
| 5. At admission to the ICU, a “checklist of risk factors” must be completed to identify patients at high risk of MDR-B carriage. |
| 6. Compliance with preventive measures including those based on transmission mechanisms should be routinely measured. |
| 7. All ICUs should develop a cleaning protocol for rooms of patients with MDR-B. |
| 8. A file/document specifying the existing equipment in the ICU and its respective cleaning protocols should be available and updated. |
| 9. To include products containing 2%–4%chlorhexidine in daily patient hygiene if colonized or infected with MDR-B. |
| 10. If an outbreak is suspected, it is recommended to identify the causative organism with molecular typing methods to know the responsible clone(s) of the outbreak and its traceability. |
Other complementary measures included an accredited online teaching module (STOP MDR-B)15 focused on epidemiological data, clinical impact, and evidences supporting bundle recommendations, diffusion of the project with presentations to all hospital services in joint sessions of the medical and nursing staff, training of ICU leaders by a coordinating team in each autonomous community, dissemination of graphic material recalling the contents of the intervention, use of posters with preventive measures or isolation and precautions at the entrance door of the rooms, empowerment of nurses to request compliance with isolation precautions by patients’ families and healthcare professionals, and daily registry of the number of isolated patients and patients with one or more MDR-B identified.
Preventive isolationOn ICU admission, barrier measures to prevent MDR-B spread were applied to patients who met one or more of the following conditions: hospital admission for more than 5 days in the previous 3 months; institutionalized patients (prison, nursing home, social health care facilities, etc.); previous MDR-B colonization or infection; use of antibiotics for more than 7 days in the previous month; presence of chronic ulcers; and hemodialysis or ambulatory peritoneal dialysis. The minimal surveillance samples to be obtained on admission and during ICU stay were oropharyngeal and/or nasal swabs and rectal swabs.
Data collectionIndividual data of all patients admitted to the participating ICUs for more than 24h during 3 months (April–June) each year were recorded using the ENVIN-HELICS database, as well as data from patients with one or more MDR bacteria monitored in the “Zero Resistance” project during the remaining 9 months annually. The “Zero Resistance” platform (http://hws.vehebron.net/resistencia-zero/RZero.asp) of the ENVIN-HELICS registry had a specific entry, protected by individual key access identifiers for each participating ICU. Data were entered on a monthly basis. The procedure of data entry is detailed in the Supplementary material (SM). Variables analyzed were those included in the ENVIN registry. The definition of each variable is described in the ENVIN registry manual (http://hws.vhebron.net/envin-helics/). Preventive contact isolation days in ICU were also collected.
OutcomesOutcomes of the “Zero Resistance” project were assessed according to the following indicators of results and/or process: a) percentage of patients with one or more MDR-B identified in samples collected within 48h of ICU admission; b) percentage of patients with one or more MDR-B acquired in the ICU in the next 48h after admission (or in the next 48h after discharge from the ICU) and c) patient-day rate in which isolation criteria were applied, defined as the number of patients-day with isolation measures per 1000 of ICU stay.
Statistical analysisMonthly data for the number of patients or days of colonizations/infections by MDR bacteria and their incidence rate ratios were analyzed. MDR-B at ICU admission was analyzed separately from ICU-acquired MDR-B. The association between the clinical impact of MDR-B (colonization or infection) and time of index culture (at ICU admission or during ICU stay) was analyzed for each family of MDR-B using the chi-square test. To estimate trends in incidence rate ratios, Poisson regression models with an offset (the number of patients or length (in days) of the ICU stay in logarithmic) were used. All tests of significance were two sided and set at p<0.05. We used R version 3.6.2 language for statistical analysis.16
ResultsA total of 103 ICUs from 15 autonomous communities participated in the “Zero Resistance” project, with a total of 139,505 patients and 833,228 days of ICU stay. Most ICUs were polyvalent (88%) from public hospitals (99.6%) with pregraduate teaching programs (70.7%), and were located in large hospitals (>500 beds) (36%) or medium-size hospitals (200–500 beds) (47%). In 51 (49.5%) ICUs, surveillance cultures were performed on admission and weekly in all patients. In 34 (33%) ICUs, surveillance cultures were performed only on admission to the population with risk factors of being MDR-B carriers and weekly in all patients. In 17 (16.5%) ICUs, surveillance cultures were performed on admission and weekly exclusively to the population with risk factors, and in 1 ICU surveillance cultures were performed on admission in all patients and weekly to the target population.
Adherence to each recommendation of the “Zero Resistance” bundle is shown in Fig. 1. A total of 10,289 MDR bacteria were identified during the study period. Of these 10,289 MDR-B, 6020 (58.5%) were isolated in 5409 patients (3.9%) on ICU admission (colonized 2.8%, infected 1.2%) and 4269 (41.5%) were new MDR-B isolated in 3648 (2.6%) patients during their stay in the ICU (colonized 1.7%, infected 1.0%). In 287 patients, MDR-B identified during their ICU stay were different than bacteria identified on admission.
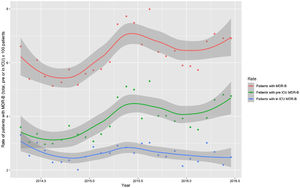
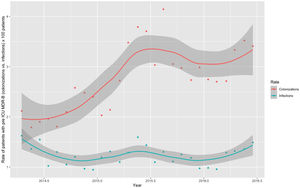
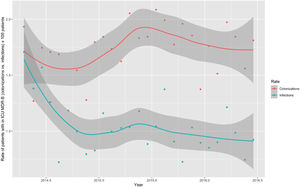
Monthly trends of MDR-B rates of patients on ICU admission and during ICU stay are shown in Fig. 2. The rate of patients with MDR-B detected on admission increased significantly, with an incidence rate ratio (IRR) of 1.43 (95% CI 1.31–1.56) over the RZ project (p<0.001), with an increase of 32.2% between the initial and final monthly rates. On the contrary, the rate of patients with MDR during ICU stay decreased, although non-significantly, with an IRR of 0.93 (95% CI 0.83–1.03) throughout the study period (p=0.174), with a 24.9% decrease between initial and final monthly rates. According to the classification into colonization or infection (Figs. 3 and 4), MDR-B colonizations showed a highly significant increase on ICU admission (IRR 1.69, 95% CI 1.52–1.83; p<0.0001) and a non-significant increase during ICU stay (IRR 1.12, 95% CI 0.98–1.29; p=0.086), meanwhile MDR-B infections showed as a non-significant decrease on ICU admission (IRR 0.95, 95% CI 0.81–1.12; p=0.549) and a very significant decrease during ICU stay (IRR 0.67, 95% CI 0.57–0.80, p<0.0001) with a 46% decrease between initial and final monthly rates.
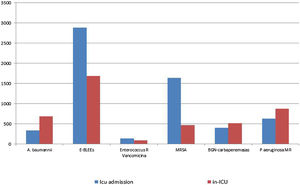
Fig. 5, shows the distribution of MDR bacteria depending on the moment of their identification. Enterobacteriacea producing extended-spectrum beta-lactamases (ESBLs) and methicillin-resistant Staphylococcus aureus (MRSA) were more frequently isolated at the time of ICU admission, whereas imipenem-resistant Acinetobacter baumannii, Pseudomonas aeruginosa and carbapenemase-producing Gram negative bacilli were more frequently isolated during ICU stay. The distribution of individual MDR-B in colonized and infected patients on ICU admission and during ICU stay is shown in Table 3. There were significant differences in the distribution in MDR-B specific infections depending on the moment of their identification, with more presence of infections by imipenem-resistant Acinetobacter baumannii, Enterobacteriaceae producing ESBLs and MRSA in MDR-B isolated during the ICU stay than at ICU admission.
Impact of multidrug-resistant bacteria (MDR-B) classified into colonization and infection.
| MDR bacteria | Total | Before ICU admission | During ICU stay | p Value |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Imipenem-resistant Acinetobacter baumannii | 1015 | 332 (32.7) | 683 (67−3) | 0.004 |
| Colonization | 681 | 243 | 438 | |
| Infection | 334 | 89 | 245 | |
| Enterobacteriaceae ESBL | 4523 | 2887 (63.8) | 1636 (36.2) | 0.016 |
| Colonization | 3094 | 2011 | 1083 | |
| Infection | 1429 | 876 | 553 | |
| Vancomycin-resistant Enterococcus spp. | 27 | 135 (59.5) | 92 (40.5) | 0.105 |
| Colonization | 186 | 106 | 80 | |
| Infection | 41 | 29 | 12 | |
| Methicillin-resistant Staphylococcus aureus | 2104 | 1636 (77.8) | 468 (22.2) | < 0.001 |
| Colonization | 1506 | 1217 | 289 | |
| Infection | 598 | 419 | 179 | |
| Carbapenemase-producing Gram negative bacilli | 918 | 400 (43.6) | 518 (56.4) | 0.919 |
| Colonization | 641 | 280 | 361 | |
| Infection | 277 | 120 | 157 | |
| Pseudomonas aeruginosa resistant to ≥3 antipseudomonal antibiotic classes | 1502 | 630 (41.9) | 872 (58.1) | 0.688 |
| Colonization | 718 | 305 | 413 | |
| Infection | 784 | 325 | 459 | |
| Total | 10,289 | 6020 (58.5) | 4269 (41.5) | <0.001 |
| Colonization | 6826 | 4162 | 2664 | |
| Infection | 3463 | 1858 | 1605 |
ESBL: extended-spectrum beta-lactamase; % is the column percentage (from the total of the column).
The rate of days of isolation for preventing MDR-B spread increased significantly during implementation of the “Zero Resistance” program with an IRR of 1.42 (95% CI 1.40–1.44) (p<0.001) and a mean of 257.58 per 1000 days of ICU stay.
DiscussionThe implementation of the “Zero Resistance” program in Spanish ICUs has been associated with a non-significant decrease in the rate of patients with MDR-B acquired during ICU stay taking together colonizations and infections, but it was associated with a very significant decrease in the rate of patients with infection produced by MDR-B acquired during ICU stay (IRR=0.67). Moreover, the “Zero Resistance” program has demonstrated that the number of MDR bacteria identified at the time of admission was significantly higher than those acquired during the patients’ ICU stay.
There are no previous reports of the implementation of a bundle for preventing MDR-B infection in the ICU setting as those recommended in the present “Zero Resistance” project. To date, control interventions alone o combined have been proposed, such as to reduce or limit excessive antibiotic prescribing and/or antibiotic cycling,17 to preform surveillance cultures for early detection of MDR-B,18–21 to use barrier precautions in patients at risk for MDR-B carrier status,22,23 and search and destroy strategies for eradication of reservoirs.24,25 Antimicrobial stewardship programs have shown to reduce antimicrobial consumption and costs26,27 as well as length of hospital stay and mortality.28 However, there is no evidence of the effect of antimicrobial stewardship programs in the prevention of MDR-B infections in ICU patients.29 The use of strategies to identify MDR-B carries has been mostly focused on MRSA.18–22,30 Results of a systematic review showed that active surveillance cultures on ICU admission and weekly thereafter reduced the incidence of MRSA infection, but the overall quality of the evidence was poor.18 In Spain, the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC) and the Spanish Society of Preventive Medicine Public Health and Hygiene (SEMPSPH) recommended systematic screening cultures for MRSA during the first hours of ICU admission.31 On the other hand, a few studies of pre-emptive contact precautions to reduce health care (HA) — associated MRSA infections have been shown to be effective in reducing the incidence of HA-MRSA transmission.32,33
In recent years, there has been also an increase of ESBL-producing and carbapenemase-producing Gram negative bacteria, multiresistant P. aeruginosa, or imipenem-resistant A. baumannii among hospitalized patients and critically ill patients admitted to the ICU.3,34 The present findings confirm that, in many cases, patients are carriers of these microorganisms on ICU admission, so that different strategies based on the presence of risk factors have been developed to support preventive isolation measures.14,35,36 However, criteria to select these patients are not sufficiently sensitive or specific, so that many patients without MDR-B are isolated and, on the other hand, MDR-B are identified in patients in which criteria are not met. Also, implementation of antibiotic optimization policies based on surveillance cultures on ICU admission and preventive isolation measures may be ineffective in the presence of MDR-B reservoirs. The need to search systematically reservoirs and to develop cleaning protocols have been extensively recommended, particularly to identify potential sources and eradication of reservoirs as key interventions to achieve termination of MDR-B outbreaks in ICUs.37
MRD bacteria were most frequently identified in surveillance samples taken on ICU admission, especially noticeable throughout the implementation period, suggesting that the main reservoir of MDR bacteria is outside the ICU, which contradicts the accepted concept of considering ICUs as the epicenter of MDR-B development.5 The active search of MDR bacteria on ICU admission was associated with a significant increase in MDR-B isolates classified as colonization with an estimated incidence rate at the end of the study 1.69 times the baseline one. It is possible that for years, many MDR-B isolated in the ICU and related to the use of antibiotics or cross-transmission would have been already present in patients on ICU admission. At the same time, whereas a significant reduction in MDR-B infections on ICU admission was not observed, ICU-acquired MDR-B infections decreased markedly throughout implantation of the “Zero Resistance” program with an IRR of 0.67. Early identification of MDR-B involves the use of products containing 2%–4% chlorhexidine in daily patient hygiene, which reduces MDR-B spread and the risk of epidemic outbreaks.38–40
A very remarkable result of the study was the detection of important differences in the distribution of MDR-B on admission and during ICU stay. ESLP-producing Enterobacteriaceae, and MRAS were especially imported to the ICUs (colonization pressure), while MDR P. aeruginosa, carbapenemase-producing Gram negative bacilli and imipenem-resistant A. baumannii, appeared preferentially during ICU stay, which suggest the presence of reservoirs for these microorganisms in the Spanish ICUs.
Limitations of the study include possible methodological differences among ICUs in surveillance sampling, bacterial culture, identification of pathogens, and susceptibility testing. Bias related to interobserver variability in the collection and registration of clinical variables cannot be excluded. Also, recommendations could have been differently applied among the participating ICUs, particularly regarding sampling strategy on admission and during ICU stay.
As a summary of the study, it can be concluded that implementation of ZR project-recommendations was associated with a non-significant decrease in the rate of patients with MDR-B acquired during ICU stay taking together colonizations and infections, but it was associated with a very significant decrease in the rate of patients with infection produced by MDR-B acquired during ICU stay. At the same time, a significant increase of patients with MDR-B on ICU admission was observed, which allowed the implementation of isolation measures to prevent dissemination. It is possible to reduce infections caused by MDR-B in the ICU by means of a multiple intervention focused on the use of antibiotics, organized search of MDR-B on admission and during ICU stay, and elimination of reservoirs of MDR-B in the ICUs.
Authors’ contributionsF. Álvarez-Lerma: project design and coordination, patient recruitment, statistical analysis, methodological review, interpretation of results, and writing of the manuscript. M. Catalán-González: project design and coordination, patient recruitment, methodological review, interpretation of results and writing of the manuscript. J. Álvarez, M. Palomar-Martínez, I. Fernández-Moreno, J. Garnacho-Montero, F. Barcenilla-Gaite, R. García, and J. Aranaz-Andrés: project design and coordination, patient recruitment, methodological review, interpretation of results. M. Sánchez-García: project design and coordination, patient recruitment, interpretation of results and writing of the manuscript. F.J. Lozano-García: project coordination, and interpretation of results. M. Martínez-Alonso: statistical analysis, methodological review, interpretation of results and writing of the manuscript. All authors have seen and approved the final draft.
Conflicts of interest and source of fundingSupported by the Spanish Ministry of Health, Social Policy and Equality through a contract (number 2014/407PN010).
We are grateful to all healthcare professionals, physicians, and nurses who participated in the “Zero Resistance” project between 2014 and 2016 with implementation of the recommendations and provision of information of the indicators analyzed in the study. All of them are coauthors of the study and their names are listed in the section of collaborators of the ENVIN registry in the annual 2014 to 2016 reports, available at http://hws.vhebron.net/envin-helics/. We are indebted to Sonia Uriona, MD, and Susana Otero for their contribution in the administration and secretariat of the ENVIN-HELICS registry and the “Zero Resistance” registry and to Marta Pulido, MD, for editing the manuscript and editorial assistance.