Anemia is the most common hematological disorder among patients admitted to the intensive care unit (ICU). The majority of critically ill patients presented with anemia upon ICU admission (up to 30% with hemoglobin <10g/dL), which tends to persist throughout the duration of their ICU stay and for many weeks after ICU discharge, unless modified by red blood cell transfusions (RBCT).1 Though the etiology of anemia of critically ill patients is multifactorial and complex, iron restricted erythropoiesis, due to absolute iron deficiency or hepcidin-induced iron sequestration with decreased iron availability (functional iron deficiency), is commonly involved in its induction and persistence.1
The most important consequence of anemia is a decreased oxygen delivery to tissues. While mild anemia does not seem to adversely affect patient's outcome, severe anemia may result in a 50% increase in the odds ratio of mortality, though RBCTs also result in a dose-dependent increase of mortality risk (up to 4-fold when more than 4 RBCT units are administered).2 Even when used with restrictive criteria, patients receiving RBCT have poorer clinical outcomes.3 Therefore, severe anemia should be avoided, corrected or, at least, ameliorated before oxygen delivery and consumption are impaired, and RBCT needed.
Additionally, non-anemic patients with reduced or absent iron stores may have symptoms such as fatigue or reduced exercise tolerance, as recently reported for patients discharged after prolonged ICU stay.4 In congestive heart failure, iron deficiency was also independently associated with compromised physical performance and quality of life, and an increase of cardiovascular and all-cause mortality; in contrast, treatment of iron deficiency with intravenous iron have been shown to improve short- and long-term functional status.5
To avoid the development and/or progression of anemia in non-bleeding ICU patients, reduction of blood losses (reduction in diagnostic phlebotomy frequency and volume, use of in-line closed blood conservation devices, cell salvage during surgical procedures, etc.) and pharmacologic stimulation of erythropoiesis should be attempted. The administration of erythropoietin emerged as a promising therapeutic option. However, when a restrictive transfusion protocol was in place, a reduction in RBCT was not consistently demonstrated, except for one randomized trial in which adjuvant intravenous iron was associated with erythropoietin.1 Failure of erythropoietin treatment in reducing RBCT may have been related to inadequate iron supplementation. Thus, laboratory definitions for absolute and functional iron deficiency, as well as doses and routes of iron supplementation should deserve especial attention.
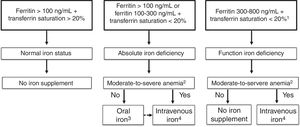
Accurate diagnosis of iron restricted erythropoiesis is essential before initiating iron supplementation. However, diagnosis of iron deficiency at ICU is difficult as ferritin levels may be elevated as part of the inflammatory response in ICU patients. A ferritin <100ng/dL indicates insufficient iron stores to support erythropoiesis in the setting of anemia and inflammation (e.g., C-reactive protein >5mg/L) which is common among ICU patients. Further markers for absolute iron deficiency are transferrin saturation <20% with ferritin concentrations of 100–300ng/mL, reticulocyte hemoglobin content <28pg, hypochromic red cells >5% or ferritin index >26 (Fig. 1). The ferritin index is the ratio between the serum-soluble transferrin receptor and the logarithm of serum ferritin.6 Ferritin concentrations >300ng/mL with transferrin saturation <20% are indicative of functional iron deficiency. These values and parameters may signal the need for intervention, as parameters of the iron status, but not anemia per se, independently influence transfusion rate, complications and in-hospital mortality7 (Fig. 1).
A tentative algorithm for management of iron deficiency in critically ill patients.
- 1.
The presence of iron deficiency can be confirmed by reticulocyte content (<28pg), percentage of hypocromic red cells (>5%), or ferritin index >2.
- 2.
Moderate-to-severe anemia: hemoglobin <11g/dL.
- 3.
Low dose (30–60mg/day) of newer oral iron formulations, such as sucrosomial iron, could be preferred. Switch to intravenous iron if intolerance to or lack of efficacy of oral iron.
- 4.
Iron sucrose (100mg/48h) or ferric carboxymaltose/iron isomaltoside/low molecular weight iron dextran (500mg/week). IVI administration should be discontinued if there is evidence of iron overload, as indicated by serum ferritin ≥1000ng/mL or transferrin saturation ≥50%.
High dose oral ferrous sulfate (325mg thrice daily; approx. 300mg elemental iron/day) has been shown to reduce RBCT requirements in critically ill patients with iron deficiency, though there was not a formal transfusion protocol.8 Recent evidence from the IRONOUT study in patients with chronic heart failure further supports the ineffectiveness of oral iron in the setting of inflammation.9 However, oral sucrosomial iron has an absorption mechanism which is mostly hepcidin-independent, and its administration at low doses (30–60mg) has been shown effective in clinical settings (e.g., chronic kidney disease) where intravenous iron seemed to be the only treatment option.10 A possible role of sucrosomial iron needs to be tested in ICU patients.
Some characteristics of available intravenous iron formulations in Spain are depicted in Table 1. The total iron dose (TID) is calculated according to: TID=body weight (kg)×2.4×hemoglobin deficiency (target hemoglobin level−patient hemoglobin level; g/dL)+500–1000mg (repletion of iron stores). For the approved indications and at recommended doses, all available “original” intravenous iron formulations are essentially equal in terms of safety and efficacy.11 However, when administering intravenous iron, more stable formulations with low labile iron content (e.g., ferric carboxymaltose or iron isomaltoside) could be preferred to avoid oxidative stress,11 though drug acquisition costs are considerably higher. Maximal single dose will depend on the available compound, but we suggested lower single dosing schedules (Table 1). Iron status should be checked weekly, and intravenous iron administration discontinued if there is evidence of iron overload, as indicated by serum ferritin ≥1000ng/mL or transferrin saturation ≥50%. In patients receiving ferric carboxymaltose, phosphate levels should be periodically monitored for early detection and management of hypophosphatemia.11
Characteristics of different intravenous iron formulations available in Spain.
| Irona sucrose | LMWIDb | Ferricc carboxymaltose | Irond isomaltoside | |
|---|---|---|---|---|
| Brand name | Venofer® | Cosmofer® | Injectafer® | Monofer® |
| Ferinject® | Monoferro® | |||
| Molecular weight (kD) | 30–60 | 165 | 150 | 150 |
| Labile iron (% injected dose)e | 3.5 | 2.0 | 0.6 | 1.0 |
| Maximal single dose (mg) | 200 | 20mg/kg | 20mg/kg (max 1000mg) | 20mg/kg |
| Suggested dosage in ICU patients: | ||||
| Dose (mg)/frequency (days) | 100/2 | 500/7f | 500/7 | 500/7 |
| Infusion time (min) | 30 | 60 | 15 | 15 |
| Maximal total dose (mg) | 2000 | 2000 | 2000 | 200 |
| Product cost per 1000mg (€)g | 112 | 103 | 192 | 192 |
Venofer summary of product characteristics. http://www.luitpold.com/documents/22.pdf (accessed 18.02.18).
LMWID, low molecular weight iron dextran; Cosmofer summary of product characteristics. http://www.cosmofer.com/product/cosmofer-spc/cosmofer-spc.aspx (accessed 18.02.18).
Ferinject summary of product characteristics. http://www.ferinject.co.uk/smpc/ (accessed 18.02.18).
The analysis of 6 randomized trials on iron supplementation in adult critical care with 860 patients found no difference in RBCT or hemoglobin, except in two trials (Table 2). These two trials involved the administration of iron isomaltoside IV to non-anemic patients at the end of cardiac surgery or oral iron to critically ill surgical patients with iron deficiency on admission. There was also no difference in secondary outcomes of mortality, in-hospital infection, or length of stay (Table 1). However, there was considerable heterogeneity between trials in study design, populations, interventions, and outcomes, and administered intravenous iron doses may have been insufficient to meet patents’ needs (Table 2). In addition, doses were substantially lower than in the REPAIR-IDA trial of intravenous iron to treat iron-deficiency anemia in non-dialysis-dependent chronic kidney disease (ferric carboxymaltose 1500mg vs. iron sucrose 1000mg), where clinically significant increments on hemoglobin levels were observed.12
Randomized controlled trials evaluating the effect of iron administration in ICU patients (6 studies, 660 patients).
| Study reference setting | Patients | Baseline Hb (g/dL) | Iron compound dose (mg) | RBCT (% or units) | Infection (%) | Mortality (%) | Hospital stay (days) |
|---|---|---|---|---|---|---|---|
| Madi-Jebara et al.a | IS:40 | 9.9 | IS (437mg) | 25% | ? | ? | ? |
| J Cardiothorac Vas Anesth 2004;18:59–63. | IS+EPO: 40 | 10.2 | IS (402mg)+EPO(300 Ukg−1) | 17% | ? | ? | ? |
| Cardiac surgery | Placebo: 40 | 10.8 | 22% | ? | ? | ? | |
| Karkouti et al.b | IS:11 | 8.5 | IS (600mg) | 18% | ? | ? | ? |
| Can J Anaesth 2006; 53:11–9. | IS+EPO: 10 | 8.3 | IS (600mg)+EPO (900 Ukg−1) | 20% | ? | ? | ? |
| Cardiac & orthopedic surgery | Placebo: 10 | 8.3 | All with oral iron after discharge | 40% | ? | ? | ? |
| Garrido-Martín et al. | IS:54 | 10.5 | IS (300mg preop+300mg postop)+oral after discharge | 37% | ? | ? | ? |
| Interact Cardiovasc Thorac Surg 2012; 15:1013–8. | 105mgday−1 preop, postop and after discharge | ||||||
| Cardiac surgery | Oral iron:53 | 10.7 | 105mgday−1 after discharge | 51% | ? | ? | ? |
| Placebo: 52 | 10.5 | 50% | ? | ? | ? | ||
| Pieracci et al.c | IS: 75 | <12 | 100mg IV thrice weekly up to 2 weeks | 73.3% | 58.7 | 9.3 | 14 |
| Crit Care Med 2014;42:2048–57. | |||||||
| Trauma ICU | Placebo: 75 | <12 | 62.7% | 69.3 | 9.7 | 16 | |
| Johanson et al.d | ISM:30 | 10.2 | ISM (1000mg at CPB end or wound closure) | 13.3% | 10 | 0 | 5 |
| Vox Sanguinis 2015; 109:257–66. | |||||||
| Cardiac surgery | Placebo:30 | 10.5 | 20.0% | 30 | 0 | 5 | |
| Litton et al.e | FCM: 70 | 8.9 | FCM 500mg/infusion | 54% | 28.6 | 7 | 15 |
| Intensive Care Med 2016; 42:1715–22 | Up to 2000mg | 79 units | |||||
| Medical and surgical ICU | |||||||
| Placebo: 70 | 8.7 | 56% | 22.9 | 4 | 18 | ||
| 121 units | |||||||
CPB, cardio-pulmonary bypass; EPO, recombinant erythropoietin; FCM, ferric carboxymaltose; FS, ferrous sulphate; Hb, hemoglobin; ICU, Intensive Care Unit; IS, iron sucrose; ISM iron isomaltoside-1000; IVI, intravenous iron; LOS, length of hospital stay; OBS, observational study; RBCT, red blood cell transfusion.
Randomization bias Hb levels: in the control group Hb were significantly higher than these observed in the treated groups.
It was estimated a priori that 20 patients in each group would be sufficient to detect an incremental increase in Hb of 0.8g/dL. A total of 3473 patients were screened. Of the 140 eligible patients, 102 refused to participate and 38 were randomized, and primary outcome was available in 31.
In subgroup analysis, also no benefit was observed for those receiving all 6 doses of iron sucrose or placebo. Follow-up 42 days or discharge.
Hb levels at postop day 5. One month after surgery, significantly more patients were non-anemic in the intravenous iron isomaltoside 1000-treated group compared to the placebo group (38.5% vs. 8.0%; p=0019).
e70% surgical patients. Inclusion criteria Hb <10g/dL, ferritin <1200ng/mL, transferrin saturation <50%. Most patients received a total of 500mg FCM. Only 15 patients received 2 doses, and only 2 patients received 3 doses.
Thus, more well-designed trials are required to ascertain which ICU patients are more likely to benefit from these treatments in terms of patient-focused outcomes, as well as to identify the optimal doses and administration schedules. Meanwhile, because of the absence of definitive clinical data, it seems reasonable to limit total intravenous iron dose to 2000mg, and to avoid its administration in patients with hyperferritinemia (>800ng/mL) or in the setting of acute infection, especially in sepsis.11
Conflict of interestsManuel Muñoz has received industry-supplied funding for consultancies, lectures and/or travel from Pharmacosmos, Vifor Pharma, Zambon, Pharmanutra, Sandoz and Celgene, and is member of the editorial board of Revista Española de Anestesiología y Reanimación and Blood Transfusion. Susana Gómez-Ramirez has nothing to declare.