Septic cardiomyopathy (SC) is an increasingly recognized condition in patients with sepsis. First described by Parker et al. in 1984, is defined as an acute onset, reversible intrinsic myocardial impairment that could cause heart failure and aggravate the whole picture.1
The incidence of myocardial dysfunction in sepsis can vary from 18 to 29% in the first 6h and could be as high as 60% later on, during the course of the syndrome.2 Mortality is high but with important variability within the studies mainly depending on their case-mix and the different diagnostic methods. In very severe cases, extracorporeal membrane oxygenation (ECMO) has been proposed as a potential cardiorespiratory support for maintaining an adequate oxygen delivery.
The aim of this “point of view” is to make a short review of its clinical features and the management strategy, including the potential role of ECMO.
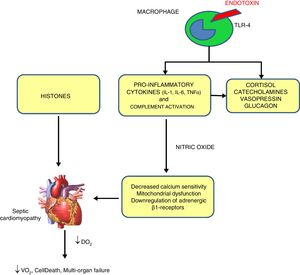
PathophysiologySeveral mechanisms have been proposed for SC,3 shown in Fig. 1.
Mechanisms of sepsis-induced cardiomyopathy. Endotoxins cause depressed cardiac contractility, which is mediated by enhanced nitric oxide (NO) production. Tumor necrosis factor and interleukin-1β also contribute to NO overproduction. NO is believed to act in the heart by decreasing myofibril response to calcium, inducing mitochondrial dysfunction, and downregulating β-adrenergic receptors. These reactions lead to sepsis-induced cardiomyopathy. Histones occur inside the nucleus and can be released into circulation because of extensive inflammation and cellular death during sepsis and are also implicated in the pathophysiology of sepsis-induced cardiomyopathy.
By exposing myocytes to plasma of septic shocked patients, many investigators reported the presence of “myocardium-depressing factors” as some of the responsible of SC. Several substances have been proposed, including TNF-α, IL-1, IL-6, complement (C5a) and endotoxin.4 Also high-circulating histone levels may be associated with left ventricular (LV) dysfunction in patients with sepsis.
Catecholamine toxicityβ-Adrenergic receptors dysfunction has been proposed as an intrinsic mechanism of SC. While β3-adrenoceptors (negative inotropic response to agonists) are upregulated by catecholamine use during sepsis, β1-receptors (positive inotropic to agonist) even when they are stimulated, they are downregulated in the next few hours after septic shock.
Decreased calcium sensitivityBeta stimulation leads to the formation of Cyclic Adenosine Monophosphate (cAMP), which in turn triggers the release of calcium ions from the sarcoplasmic reticulum so contraction of myofibrils begins. During sepsis, phosphorylation of troponin complex decreases its calcium sensitivity and reduces myocardial contraction.
Nitric oxide (NO)This ubiquitous and multifunctional compound can be produced by constitutive and/or inducible NO synthases. Inducible NO synthases specially NOS-2 are increased in septic humans leading to increased levels of NO which in turn can cause indirect oxidative injury made by free oxygen radicals and its nitrous derivatives and decreased mitochondrial activity and glutathione depletion.
Clinical characteristics and diagnosisThe main features of SC are the absence of coronary occlusion and is transience. SC usually appears within the first few hours of septic shock, then there is a progressive return toward a normal or previous Ejection Fraction (EF) in 7–10 days after the onset of sepsis.5 In this setting, systolic dysfunction may be evaluated by two-dimensional speckle tracking echocardiography (STE), due to its higher sensitivity than conventional echocardiography.
Clinical features and echocardiography:
- •
Clinical signs of heart failure: Tachycardia, persistent hypotension after norepinephrine, elevated lactate levels and low SvO2 or ScvO2.
- •
Slight LV dilatation with normal or low filling pressure due to increased LV compliance and end-diastolic volume, associated with a reversible hypocontractility, as described in the seminal publication by Parker.1
- •
Despite of global ventricular dysfunction and low EF, stroke volume remains preserved in the early phase.
- •
Catecholamines may unmask SC by increasing systemic vascular resistance, causing decreased Cardiac Index (CI).
- •
Takotsubo cardiomyopathy is very infrequent.
- •
Right ventricular (RV) dysfunction could be present in 30% of the patients. Severe RV dysfunction (free wall strain) assessed by STE may be associated with a worse prognosis.
Troponin elevation is very specific and reflects myocardial injury. Troponins are elevated in 31% to 85% of patients with severe sepsis probably by reversible damage to intracellular organelles. Troponin elevation is associated with a higher risk of death among patients with sepsis,6 being useful to establish which patients could benefit of strict hemodynamic and cardiac assessment during the first hours of shock. B-type Natriuretic Peptide (BNP) is also elevated in patients with SC but renal impairment can reduce the clearance of natriuretic peptides. In this clinical context, it seems reasonable to rule out severe cardiac impairment when a septic patient has low BNP levels while any other value should trigger further evaluation.
TreatmentTreatment strategies are based in achieving a balanced DO2/VO2, basically by increasing CI up to correct tissue dysoxia and reducing VO2 with analgosedation+/− neuromuscular blockade, mechanical ventilation and antipyretics.
InotropesCurrent guidance recommends using dobutamine to increase the CI in patients who show evidence of persistent hypoperfusion despite adequate fluid loading and the use of vasopressor agents. However, routinely increasing CI to predetermined “supranormal” levels in all patients clearly does not improve outcomes.
Alternative inotropic agents might be used to increase cardiac output in specific situations. The phosphodiesterase inhibitor, milrinone, was shown to increase cardiac output in one small randomized trial. Levosimendan increases cardiac myocyte calcium responsiveness and also opens ATP-dependent potassium channels, giving the drug both inotropic and vasodilatory properties. It may be especially useful for patients with right ventricle dysfunction. However, trials comparing levosimendan with dobutamine show no clear advantage for levosimendan, and it is more expensive than dobutamine.
Extracorporeal membrane oxygenation (ECMO)In recent years, positive experiences using ECMO as respiratory support with a venovenous cannulation strategy (VV ECMO) and as cardiac/cardiorespiratory support with a venoarterial strategy (VA ECMO) have been reported. In patients with refractory septic shock, the support with ECMO for maintaining adequate DO2 has been considered.
The currently available ECMO circuits offer biocompatible coating for all the surfaces that come into contact with the blood, and thus limit to some extent its biological response. However, the initiation of the support triggers a release of proinflammatory mediators and activates the coagulation pathway, similar to the underlying pathophysiological mechanisms triggered in sepsis. For these reasons, coupled with the theoretical possibility that circulating microorganisms may become lodged in the inert structures of the circuit, sepsis was initially considered as a contraindication for initiating ECMO support. However, in septic patients ECMO may optimize tissue perfusion, allowing a “metabolic rest” by reducing the need for pressor and inotropic drugs and enabling the use of less aggressive ventilatory support. Five recent retrospective studies have offered some insights regarding the use of ECMO in adult patients with refractory septic shock7–11 (see description in Table 1).
Summary of characteristics of interest of publications on the use of ECMO support in adult patients with refractory septic shock.
| Study | N | Period | Cannulation | Hospital survival | CPR preECMO | Factors associated with higher mortality |
|---|---|---|---|---|---|---|
| Bréchot (2013) | 14 | Jan 2008 Sept 2011 | Peripheral VA 36% switch to VV | 71% | 0 | – |
| Huang (2013) | 52 | Jan 2005 Dec 2010 | Peripheral VA | 21.9% | 40% | Age>60 years |
| Cheng (2013) | 108 | Jan 2001 Dec 2009 | Peripheral VA 80% Peripheral VV 20% | 29% | 29% | 1. CPR during ECMO 2. Age>55 years 3. History of stroke 4. Post ECMO neurologic deficit 5. Dialysis dependence |
| Park (2015) | 32 | Jan 2005 Dec 2013 | Peripheral VA | 15% | 43.8% | 1. CPR pre ECMO 2. Low troponin I peak 3. High SOFA score at day 3 |
| von Bahr (2017) | 255 | 1995 Dec 2013 | Peripheral VV 68% 21% switch to VA Peripheral VA 33% 16% switch to VV | 64% | 0 | - |
CPR: cardiopulmonary resuscitation; Dec: December; ECMO: extracorporeal membrane oxygenation; Jan: January; Sept: September; VA: venoarterial; VV: venovenous.
According to the analysis of these studies we can infer the subgroup of patients who would benefit most from ECMO support. Patients with predominantly low flow secondary to SC1 may be good candidates for VA ECMO life support. In contrast, in patients with a pattern of distributive shock with high cardiac output and low systemic vascular resistance, ECMO appears to achieve worse results, and is considered a controversial indication. ECMO should not be indicated in septic patients who have suffered a cardiorespiratory arrest resulting from shock, due to the very poor results obtained.
Another point of interest is the use of VV ECMO in those patients with compromised hemodynamic status. In the early stages of hemodynamic compromise in the septic patient, right ventricular dysfunction plays an important role. VV ECMO could improve right ventricular function by contributing to correction of hypoxemia, acidosis and pulmonary hyperinflation. After initiation of the support with this cannulation strategy, there is usually a notable hemodynamic improvement in which the reduction of pulmonary vascular resistance, better myocardial oxygenation, better control of body temperature and a decrease in airway pressures all play a vital role.
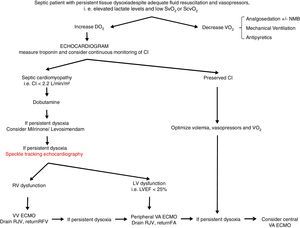
From a practical point of view (Fig. 2), an echocardiogram is essential in refractory shock in sepsis to evaluate biventricular function before starting ECMO support. If the hemodynamic situation is attributed to right ventricular dysfunction, VV support could be indicated, while if it is due to a clear left ventricular dysfunction, VA should be used. In intermediate situations, VV cannulation with drainage cannula in the right jugular vein and return cannula in the right femoral vein might be a reasonable option, with continuous evaluation of hemodynamic changes and oxygen transport balance. If minimal stability is not achieved, the return cannula should be changed to the femoral artery for a VA ECMO support.
Proposed algorithm for Management of myocardial dysfunction in septic shock. CI: Cardiac Index; DO2: oxygen transport; ECMO: extracorporeal membrane oxygenation; FA: femoral artery; LV: left ventricle; LVEF: left ventricular ejection fraction; NMB: neuromuscular blockers; RFV: right femoral vein; RJV: right jugular vein; RV: right ventricle; SvO2: venous saturation; ScvO2: central venous saturation; VO2: oxygen consumption; VA: venoarterial; VV: venovenous.
The incidence of SC could be as high as 60%, with high mortality. Treatment strategies are based in achieving a balanced DO2/VO2, basically in increasing cardiac output with inotropes up to correct tissue dysoxia. For those patients with refractory cardiovascular dysfunction, ECMO might be considered as a valuable therapeutic option, although more data are needed to confirm the current evidence.
Financial supportThe authors have not received any financial support in relation with this manuscript.
Conflict of interestThe authors declare that they do not have conflict of interest related to this manuscript.