Enterovirus (EV) is a common cause of infection in newborns and infants younger than 3 months.1 Its course can be variable, from a self-limited fever to fulminant hepatitis, sepsis or meningoencephalitis. It may also debut as acute fulminant myocarditis with cardiovascular collapse, secondary to incessant arrhythmias or due to fast hemodynamic deterioration.2 This cardiogenic shock may be refractory to conventional support (mechanical ventilation and vasoactive drugs), leading to a high risk of mortality3 and poor prognosis.4 In recent years, veno-arterial extracorporeal membrane oxygenation (VA-ECMO) has become a major life-saving strategy in refractory cardiac failure.5 However, data regarding ECMO use in newborns affected by EV myocarditis are limited6 and its use is controversial due to low survival rates and potentially irreversible myocardial damage.
We present two cases with EV myocarditis, all of them males. Main data can be seen in Table 1. Both of them were infected during EV season (summer–autumn) and developed symptoms before 15 days of life during 15 days of life. None of the mothers presented symptoms.
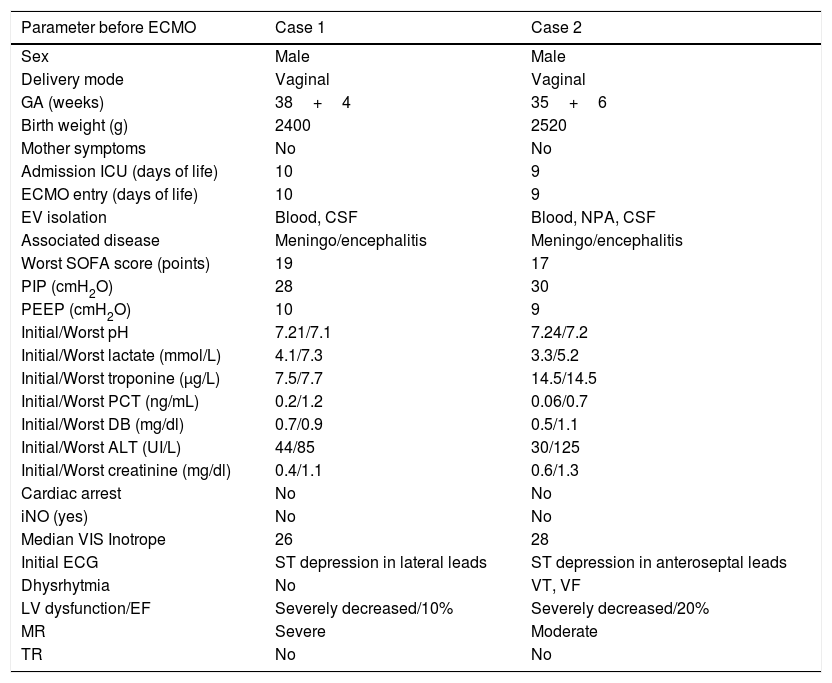
Demographic data and clinical parameters before ECMO.
| Parameter before ECMO | Case 1 | Case 2 |
|---|---|---|
| Sex | Male | Male |
| Delivery mode | Vaginal | Vaginal |
| GA (weeks) | 38+4 | 35+6 |
| Birth weight (g) | 2400 | 2520 |
| Mother symptoms | No | No |
| Admission ICU (days of life) | 10 | 9 |
| ECMO entry (days of life) | 10 | 9 |
| EV isolation | Blood, CSF | Blood, NPA, CSF |
| Associated disease | Meningo/encephalitis | Meningo/encephalitis |
| Worst SOFA score (points) | 19 | 17 |
| PIP (cmH2O) | 28 | 30 |
| PEEP (cmH2O) | 10 | 9 |
| Initial/Worst pH | 7.21/7.1 | 7.24/7.2 |
| Initial/Worst lactate (mmol/L) | 4.1/7.3 | 3.3/5.2 |
| Initial/Worst troponine (μg/L) | 7.5/7.7 | 14.5/14.5 |
| Initial/Worst PCT (ng/mL) | 0.2/1.2 | 0.06/0.7 |
| Initial/Worst DB (mg/dl) | 0.7/0.9 | 0.5/1.1 |
| Initial/Worst ALT (UI/L) | 44/85 | 30/125 |
| Initial/Worst creatinine (mg/dl) | 0.4/1.1 | 0.6/1.3 |
| Cardiac arrest | No | No |
| iNO (yes) | No | No |
| Median VIS Inotrope | 26 | 28 |
| Initial ECG | ST depression in lateral leads | ST depression in anteroseptal leads |
| Dhysrhytmia | No | VT, VF |
| LV dysfunction/EF | Severely decreased/10% | Severely decreased/20% |
| MR | Severe | Moderate |
| TR | No | No |
CSF: cerebrospinal fluid. DB: direct bilirubin. ECMO: extracorporeal membrane oxygenation implantation. EF: ejection fraction. GA: gestational age. ICU: intensive care unit. EV: enterovirus. iNO: inhaled nitric oxide. LV: Left ventricular. MR: mitral regurgitation. NPA: nasopharyngeal aspirate. PCT: procalcitonin. PEEP: positive end-expiratory pressure. PIP: positive inspiratory pressure. SOFA: Sepsis Organ Failure Assessment score. SVT: supraventricular tachycardia. TR: tricuspid regurgitation. VIS: vasoactive-inotropic score. VF: ventricular fibrillation. VT: ventricular tachycardia.
Both patients were admitted to the Intensive Care Unit with the initial suspicions of septic shock but echocardiography and ECG along with cardiac biomarkers guide to myocarditis diagnosis. In the presence of a patient with myocarditis, an infectious extension study is carried out with the collection of samples for multiple viruses and bacteria. The diagnosis of EV infection was made by positive EV RNA with real-time PCR (NucliSENS®easyMag®, bioMérieux, Marcy l’Etoile, France) in the different isolation sites.
The two patients presented ST depression in the initial ECG. Second case presented ventricular tachycardia and ventricular fibrillation. Both had a severe left ventricular dysfunction with less than 20% of ejection fraction and severe mitral regurgitation.
Initial and worst pH, lactate, troponine, and procalcitonin levels, and renal and hepatic function of the patients are summarized in Table 1.
Due signs of insufficient organ perfusion and repeated runs of tachycardia although conventional inotropic and respiratory management, veno-Arterial ECMO was initiated.
Approximately 80–100% of the cardiac output was assisted on ECMO being both of them treated with inodilatador agents, levosimendan and milrinone, during ECMO therapy.
One patient required balloon atrial septostomy at 2h of ECMO support to decompress the left ventricle. Complications from ECMO included circuit change due to clots and arrhythmias that required medical treatment.
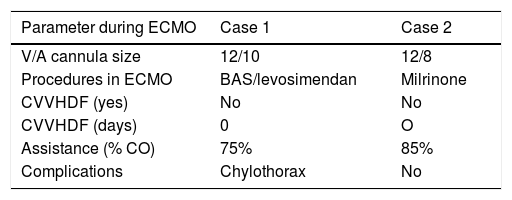
Data about days of mechanical ventilation, ICU and hospital length of stay, and neurological and cardiac outcomes of the all patients are summarized in Table 2. Both received intravenous immunoglobulin (1g/kg for three consecutive days).
Summary of parameter during ECMO and outcomes.
| Parameter during ECMO | Case 1 | Case 2 |
|---|---|---|
| V/A cannula size | 12/10 | 12/8 |
| Procedures in ECMO | BAS/levosimendan | Milrinone |
| CVVHDF (yes) | No | No |
| CVVHDF (days) | 0 | O |
| Assistance (% CO) | 75% | 85% |
| Complications | Chylothorax | No |
| Evolution | ||
|---|---|---|
| ECMO (days) | 10 | 9 |
| MV (days) | 15 | 16 |
| ICU (days) | 20 | 25 |
| Hospital LOS (days) | 30 | 35 |
| Neurological disability (Yes) | No | No |
| Last echocardiography | Mild biventricular dysfunction, EF 48% | Normal biventricular function, EF 55% |
| Outcome | 6 months of life | 12 months of life |
| Survival | Yes | Yes |
BAS: balloon atrial septostomy. CO: cardiac output. CVVHDF: continuous veno-venous hemodiafiltration. ECMO: extracorporeal membrane oxygenation. EF: ejection fraction. ICU: intensive care unit. LOS: hospital length of stay. LV: left ventricular. MV: mechanical ventilation. V/A: veno/arterial.
Survival was 100% and they recovered biventricular function after ECMO. None of them has neurological disabilities today.
In our retrospective study, we report a survival rate higher than that previously described in the literature (100%).7 Therefore although literature survival rates in newborns affected by EV myocarditis are low (33–50%)3,7 and ECMO support remains controversial, we suggest ECMO support in those patients with high mortality rate expectancy: patient survival and full recovery are possible.
EV may cause inflammatory disease of the myocardium associated with myocyte necrosis, leading to fulminant myocarditis and consequently congestive heart failure, dysrhythmias and cardiogenic shock.8 This dysfunction might be reversible. The main problem is the situation of refractoriness of the shock that cannot be sustained with the usual ICU measures, so that patients evolve to very precarious conditions, even to irreversible multiorgan dysfunction and/or cardiac arrest.2 Clinical myocarditis can mimic other clinical patterns. ECMO is a life-saving therapy that is able to restore hemodynamics while allowing for possible recovery. However, patients on ECMO support may develop severe complications including neurologic, bleeding and thrombosis and infection among others.9 Therefore, the indication of ECMO in these patients can be a challenge, especially their weaning from ECMO support: longer ECMO executions have been associated with poor outcomes.10
Arrhythmias may be found in 25–70% of the patients with EV myocarditis.7–8 The use of high inotropic dosage increases myocardial oxygen consumption and exacerbates these rhythm disturbances. The use of ECMO restores patient hemodynamics, allowing the rapid withdrawal of inotropics and decreasing the incidence of subsequent arrhythmias.8
One of the strategies to promote left heart recovery while patients are on VA-ECMO is to decompress the left ventricle through balloon atrial septostomy or left ventricular venting. This strategy avoids the myocardial ischemia due to left-ventricular high end-diastolic pressures and also ameliorates the pulmonary edema secondary to left-atrial hypertension. In our sample, one patient did not require septostomy due to a patent foramen ovale. Left-to-right shunt might have helped to decrease end-diastolic pressure, favouring recovery and minimizing the pulmonary edema. Also appropriate afterload reduction with the use of inodilators such as milrinone and levosimendan is key to improving left ventricular stroke volume and promoting effective decompression.
In previous studies8 in which ECMO was used in neonatal EV myocarditis patients, the average duration was 7 days; in our experience it was 9 and 10 days, with a complete recovery in both and 100% survival. If ECMO support had not been initiated in the situation of hemodynamic refractoriness to conventional treatment, we expected a higher mortality rate. Inwald et al. in 20043 reported 7 patients with EV myocarditis: three did not receive support with ECMO, and all survived; the 4 who required ECMO, the mortality rate was 75%. However, the small sample of both studies precludes us from being emphatic in our conclusions.
We believe that more studies about ECMO in EV myocarditis are required to draw stronger conclusions about ECMO viability. However, we believe that our data are encouraging, since the reported mortality of these patients without ECMO. Our results reveal that survival can be achieved without neurological damage. As ECMO support is improving, complications, although they may be severe, are becoming less frequent.