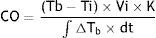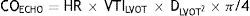Invasive cardiac monitoring using thermodilution methods such as PiCCO® is widely used in critically ill patients and provides a wide range of hemodynamic variables, including cardiac output (CO). However, in post-cardiac arrest patients subjected to therapeutic hypothermia, the low body temperature possibly could interfere with the technique. Transthoracic Doppler echocardiography (ECHO) has long proved its accuracy in estimating CO, and is not influenced by temperature changes.
ObjectiveTo assess the accuracy of PiCCO® in measuring CO in patients under therapeutic hypothermia, compared with ECHO.
Design and patientsThirty paired COECHO/COPiCCO measurements were analyzed in 15 patients subjected to hypothermia after cardiac arrest. Eighteen paired measurements were obtained at under 36°C and 12 at ≥36°C. A value of 0.5l/min was considered the maximum accepted difference between the COECHO and COPiCCO values.
ResultsUnder conditions of normothermia (≥36°C), the mean difference between COECHO and COPiCCO was 0.030 l/min, with limits of agreement (−0.22, 0.28) – all of the measurements differing by less than 0.5 l/min. In situations of hypothermia (<36°C), the mean difference in CO measurements was −0.426 l/min, with limits of agreement (−1.60, 0.75), and only 44% (8/18) of the paired measurements fell within the interval (−0.5, 0.5). The calculated temperature cut-off point maximizing specificity was 35.95°C: above this temperature, specificity was 100%, with a false-positive rate of 0%.
ConclusionsThe results clearly show clinically relevant discordance between COECHO and COPiCCO at temperatures of <36°C, demonstrating the inaccuracy of PiCCO® for cardiac output measurements in hypothermic patients.
La monitorización invasiva cardiaca mediante métodos de termodilución, como PiCCO®, es ampliamente utilizada en pacientes críticamente enfermos y proporciona una gran variedad de variables hemodinámicas, como el gasto cardiaco (GC). No obstante, en los pacientes post-paro cardíaco bajo hipotermia terapéutica, la baja temperatura corporal podría interferir con la técnica. La ecocardiografía doppler transtorácica (ECHO) ha demostrado su exactitud en la estimación del GC y no está influenciada por los cambios de temperatura.
ObjetivoEl objetivo del presente estudio fue evaluar la exactitud de PiCCO® para medir el GC en pacientes bajo hipotermia terapéutica, en comparación con ECHO.
Diseño y pacientesSe analizaron 30 pares de mediciones GC_ECHO/GC_PiCCO en 15 pacientes sometidos a hipotermia después de un paro cardíaco. La máxima diferencia aceptada entre los valores de GC_ECHO y GC_PiCCO se consideró 18 mediciones pareadas se realizaron a menos de 36°C y 12 a ≥36°C. 0,5L/min.
ResultadosEn la normotermia (≥36°C), la diferencia media entre GC_ECHO y GC_PiCCO fue de 0,030L/min, con límites de concordancia (–0,22; 0,28), todas las medidas difieren menos de 0,5L/min. En la hipotermia (<36°C), la diferencia media de las mediciones fue –0,426L/min con límites de concordancia (–1,60; 0,75) y solo el 44% de las mediciones cayeron en el intervalo (–0,5; 0,5). El límite de temperatura calculado que maximiza la especificidad fue 35,95°C, por encima del cual la especificidad fue del 100% y la tasa de falsos positivos del 0%.
ConclusionesLos resultados muestran claramente una discordancia clínicamente relevante entre GC_ECHO y GC_PiCCO en temperatura <36°C, lo que revela la inexactitud de PiCCO® para las mediciones del gasto cardíaco en pacientes hipotérmicos.
In post-cardiac arrest patients, mild induced hypothermia has proven to be neuroprotective and to improve the global outcome after the initial period of cerebral hypoxia-ischemia.1,2 This probably occurs because hypothermia reduces cerebral oxygen demand, decreases intracranial pressure and also limits the production of oxygen free radicals, diminishing brain damage.1 The term targeted temperature management is now preferred, and the most recent resuscitation guidelines recommend a constant temperature between 32–36°C for at least 24h, although the optimal target temperature remains uncertain, waiting for more large controlled trials on this matter.3
The initial management of the so called ‘post-resuscitation syndrome’ is challenging. Hypovolemia, excessive vasodilation and reversible myocardial stunning frequently results in early hypotension that can be life-threatening.4,5 This hemodynamic instability is managed with the use of fluids, inotropes and vasopressors if needed.3,4 Therefore, it is important to have a correct monitoring of hemodynamic and pulmonary variables, in order to optimize those therapies,5 sometimes using invasive devices.
The PiCCO® (pulse index continuous cardiac output) system, in use for over 10 years, allows the measuring of a large number of variables throughout central venous and peripheral arterial catheterization alone. Among other parameters, it is used to measure cardiac output (CO) through a transpulmonary thermodilution method.
Despite the unquestionable utility of PiCCO® in situations of hemodynamic instability, as a thermodilution method, it is assumed that the temperature within the artery stays stable during calibration and measurements. That might not be the case during hypothermia and other variations of body temperature.
Transthoracic Doppler echocardiography (ECHO) has long proved its accuracy in CO estimation, including in critically ill patients.6–10 It can be performed in different scenarios, including therapeutic hypothermia, and it is not influenced by temperature changes.
Therefore, the aim of the present study was to assess the concordance between the CO values measured by PiCCO® (COPiCCO) and by ECHO (COECHO), in patients under therapeutic hypothermia following cardiac arrest.
MethodsStudy design and patientsThis single-center, prospective cohort study was conducted in the intensive care unit (ICU) of a tertiary hospital of Lisbon, between August 2014 and July 2015. Following approval of the ethic committee of Centro Hospitalar de Lisboa Central, informed consent was obtained from each patient's next of kin or a posteriori from the patient himself.
The study included all patients admitted to the ICU for therapeutic hypothermia following cardiac arrest, according to the unit protocol. The exclusion criteria were any condition that contraindicated the insertion of PiCCO® or impaired the transthoracic echocardiographic window, as neck and thoracic severe trauma, burning or skin infection. Additionally, patients were excluded if they had pathology that could diminish the accuracy of CO calculation using ECHO (chronic atrial fibrillation, cardiac valve disease, pulmonary thromboembolism or intracardiac shunts) or PiCCO (severe peripheral arterial disease or arterial bypass).
ProceduresAt ICU admission, patients received central venous catheter (Kimal®; 5-lumened, 8.5Fr×15cm) on the internal jugular or subclavian vein and a PiCCO® catheter (Pulsion Medical Systems, 5Fr×20cm) inserted in the femoral artery, connected to a Siemens MP 50 monitor with the appropriate software. Based on the modified Stewart-Hamilton equation, cardiac output is inversely related to the concentration and total passage time of an indicator solution measured after its transit through the heart.11
Equation 1: Adapted Stewart-Hamilton equation, where CO=Cardiac output; Tb=Blood temperature; Ti=injectate temperature; Vi=injectate volume; K=constant; ∫ΔTb×=area under the thermodilution curve.During the therapeutic hypothermia and rewarming period, CO was calculated through the PiCCO system followed by ECHO estimation (General Electric® VIVID S5, with 3.5MHz probe). The two measurements were performed by different, blinded investigators, as close as possible (maximum interval of time: 10minutes). At the same time, central temperature and hemodynamic data (heart rate, rhythm and mean arterial systemic pressure) were registered. It was assured the constancy of each interfering variable such as level of sedation, ventilation parameters, vasoactive drugs or any other IV infusion. Therefore, a pair of CO measurements (one by PiCCO and one by ECHO) for a determined temperature was obtained. Additionally, demographic data, severity scores at admission, cause and rhythm of arrest and final outcome were collected.
We considered 0.5L/min as the maximum clinically accepted difference between the values of COECHO and COPiCCO. In other words, the two methods were considered to agree when the difference fell in the interval (−0.5, 0.5).
PiCCO measurementsFor the CO calculation by thermodilution technique, 20ml of iced (<8°C) saline 0.9% was delivered in the proximal lumen of the central venous catheter and mixed with blood through the systemic and pulmonary circulation. The injection was performed as rapidly as possible, irrespective of the respiratory cycle. The thermistor-tipped arterial line, placed in the femoral artery, quantified the change in temperature over time. The thermodilution curve recorded by the arterial thermistor was automatically analyzed by the PiCCO® software, obtaining the value of CO. Triplicate injections were performed for each set of measurements and considered the mean value of the three.
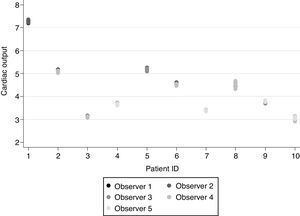
Echocardiographic measurementsThe Doppler estimated CO was obtained multiplying the heart rate (HR) by the Doppler estimated stroke volume (SV). The last one uses the velocity-time integral of flow through the left ventricular outflow tract (VTILVOT) and the area of the left ventricular outflow tract (ALVOT) which, in turn, is calculated using the left ventricular outflow tract diameter (DLVOT) by the following formula:
The VTILVOT was recorded by pulsed-wave Doppler from an apical fiver chamber view, by placing the Doppler sample immediately below the aortic valve annulus, aligned with the center of the valve where the flow is maximum. The final value of VTILVOT was the mean of three consecutive determinations. DLVOT was measured in a parasternal long-axis view, just before the aortic annulus, from the inner edge to outer edge, parallel to the valve apparatus.
Five complete and consecutive measurements were performed, and considered the mean as the final value for COECHO.
Echocardiography inter-observer and intra-observer variability analysisIn order to assure the reliability of the COECHO measurements, as the operator dependent errors are the major pitfall of this technique, a previous study was conducted to evaluate the echocardiographic skills of the five investigators involved in the major study. Repeating the procedure as described above (mean three determinations of VTILVOT and one of DLVOT), each investigator performed five COECHO measurements in a patient under therapeutic hypothermia and was followed by other blinded investigator who obtained another five measurements. This procedure was repeated in ten different patients so that every investigator was compared with each of the other four.
Statistical analysisContinuous variables were described with mean and standard deviation (SD) or median and inter-quantile range (IQR: 25th percentile–75th percentile), as appropriate. Categorical data were presented as frequencies and percentages.
To assess the precision of the ECHO measurements, the intra-observer repeatability and inter-observer reproducibility were studied using intraclass correlation coefficients (ICCs), and corresponding 95% confidence intervals. These were estimated using a generalized linear mixed effects model with a random intercept and a random slope, taking into account the correlation structure between CO measures of the same patient. To evaluate the agreement between ECHO and PiCCO for measuring CO, Bland–Altman graphical method was used. Additionally, a stratified analysis by temperature (<36°C, ≥36°C) was performed using generalized linear mixed effects models with a random intercept, where the difference between the two methods was adjusted by temperature. Both generalized linear mixed effects models considered a variance–covariance matrix defined as a multiple of the identity matrix.
The best cut-off value that identifies patients who have a difference between COECHO and COPiCCO belonging to the interval (−0.5, 0.5), was calculated by maximizing specificity.12 After discretizing CO measures by the obtained cut-off value, diagnostic test performance measures (sensitivity, specificity, positive and negative predictive values as well as false positive and false negative rates) were calculated.
The level of significance α=0.05 was considered. Data analysis was performed using Stata (StataCorp. 2011; Stata Statistical Software: Release 12, College Station, TX: StataCorp LP.) and R software (R: A Language and Environment for Statistical Computing, R Core Team, R Foundation for Statistical Computing, Vienna, Austria, year=2014, http://www.R-project.org.).
ResultsPatientsFifteen patients met the inclusion criteria of the study. The demographic characteristics, severity scores at admission as well as the arrest rhythm and cause are displayed in Table 1. Eleven patients (73%) initiated hypothermia protocol in the ICU, one started cooling in pre-hospital care and the remaining three began the protocol in the Emergency Department. In-hospital mortality was 47%.
Patients’ characteristics.
| Patients’ characteristics (n=15) | |
|---|---|
| Age median (IQR) years | 66 (57–69) |
| Male gender n (%) | 11 (73) |
| Caucasian n (%) | 11 (73) |
| Severity scores | |
| APACHE II (median) | 23 |
| SAPS II (median) | 58 |
| Arrest rhythm n (%) | |
| Asystole | 7 (47) |
| Ventricular fibrillation | 3 (20) |
| Unknown | 5 (33) |
| Arrest cause n (%) | |
| Cardiogenic shock | 4 (26) |
| Septic shock | 1 (7) |
| Hypovolemic shock | 1 (7) |
| Respiratory insufficiency | 3 (20) |
| Metabolic coma | 1 (7) |
| Unknown | 5 (33) |
Regarding the intra-observer repeatability and inter-observer reproducibility, the intraclass correlation coefficients were both equal to 0.998 (95% CI: 0.995–0.999) (Fig. 1).
CO measurementsA total of 30 paired COECHO/COPiCCO measurements were analyzed. The mean time interval between the measurements was 7 (SD=2)minutes. Mean temperature during the measurements was 35°C, with 18 paired measurements performed under 36°C and 12 at a temperature equal or superior to 36°C.
In overall pairs of CO measurements, the mean difference between the two methods was −0.24L/min, with limits of agreement (−1.26, 0.77).
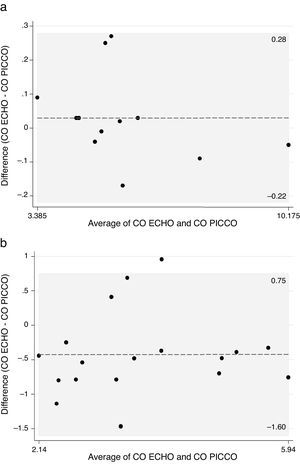
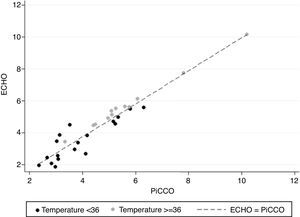
Considering the measurements at normothermia (≥36°C), the mean difference was 0.030L/min, with limits of agreement (−0.22, 0.28). All of the 12 pairs of CO measurements registered at this temperature, differed less than 0.5L/min, previously defined as an acceptable difference (Fig. 2a).
In hypothermia (<36°C), the mean difference of CO measurements was −0.426L/min with limits of agreement (−1.60, 0.75) and only 44% (8/18) of the paired measurements fell in the interval (−0.5, 0.5). For the remaining 10 measurements that differed more than 0.5L/min, the difference COECHO−COPiCCO was negative in most cases (Fig. 2b).
Fig. 3 reinforces that the concordance between COPICCO and COECHO measurements was better when temperature was equal or above 36°C.
The calculated temperature cut-off, in order to maximize specificity, was 35.95°C. Above this temperature, the specificity was 100% and the false positive rate was 0%, traducing a complete agreement between the methods.
Results of the linear mixed effects models (Table 2) are in accordance with those obtained previously. In fact, for temperatures <36°C the difference between the two methods, after adjusting for temperature, was statistically significant (p=0.001). However, for temperatures ≥36°C, no significant difference was found between the two methods (p=0.374). Additionally, the resulting ICC of the model for temperatures higher or equal 36°C was higher (0.998, 95% CI: 0.993–0.999) than that obtained for lower temperatures (0.843, 95% CI: 0.652–0.939).
Multivariable regression analysis results for CO values stratified by temperature.
The results clearly show that the discordance between the cardiac output measured by PiCCO and transthoracic echocardiogram is bigger at lower temperatures. Comparing the paired measurements during hypothermia (<36°C) and normothermia (≥36°C), it is clear that the later ones are far more coincident, as shown in Fig. 2 and confirmed by the results of the multivariable analysis. The control of potential confounding variables was assured for every pair of measurements, thus any difference between COECHO and COPiCCO can be attributed exclusively to the methods. Furthermore, as echocardiography is not influenced by temperature changes, we concluded that lower body temperature diminishes accuracy of PiCCO measurements. This interference is called “thermal noise”, and several authors proved its influence on the precision and accuracy of thermodilution measurements.13–16
To evaluate a certain measurement method, one should consider both precision – closeness of agreement between replicate measurements – and accuracy – closeness of agreement between a measurement value and its true value.17 Tagami et al. studied the precision of PiCCO in hypothermic patients following cardiac arrest by comparing measurements at different temperatures, during hypothermia and the rewarming period and found no significant changes.5 However, as Gasparetto et al. pointed out, not only the precision or reproducibility of PiCCO is important to validate but also its accuracy18 under this condition. This means responding to the question “how close the measurement is to the actual or real value” and implies a comparison with a gold standard technique, in this context, ideally non temperature-dependent, such as echocardiography.
On interpreting the results, it must be remembered that echocardiography, itself, has its limitations and is not exempt of errors. Concretely in the obtainment of cardiac output values, it requires expertise on performing the five chamber view to measure VTILVOT and the long axis view, sometimes difficult in the critically ill patients, to measure the left ventricular outflow tract diameter (DLVOT). Errors in this latest parameter are particularly serious, as it is squared in the CO formula. To overcome the potential errors on measuring the DLVOT, Vermeiren et al. suggests its calculation according to the body surface area (DLVOT=5.7×BSA+12.1). The authors also admit the use of fixed values for DLVOT like 1.8 for female and 2.0 for male patients, or even the general value of 3cm2 for ALVOT, which would greatly simplify the conclusions and decisions at the bedside.19
The measurements performed at ≥36° had a very small mean difference (0.03L/min) with narrow limits of agreement (−0.22, 0.28), therefore considered completely concordant, in terms of clinical practice.
On the other hand, the measurements of CO during hypothermia (<36°C) had a mean difference of 0.426L/min, still within the interval considered clinically accepted, but with much wider limits of agreement (−1.60, 0.75), traducing the elevated number of measurements that differed >0.5L/min. In fact, more than 50% of the paired measurements performed at <36°C differed more than 0.5L/min, with a tendency to overestimate CO measured by PiCCO.
Accordingly, the temperature cut-off value that assured specificity of 100% was 35.95°C, virtually the same as the 36°C used to distinguish normothermia and hypothermia. This means that only above this temperature the difference COECHO−COPiCCO was <0.5L/min for all paired measurements.
In clinical practice, this can be extremely relevant, as the difference between COECHO−COPiCCO that we considered as clinically accepted (0.5L/min) is already rather high in the context of critically ill and often hemodynamically instable patients. As so, a method that misleads the CO calculation, by more than 0.5L/min, seems to us too inaccurate to be useful in these situations.
The implications of these findings can overtake the strict context of hypothermia after cardiac arrest, as the PiCCO system is used in other situations of low body temperature. For example patients experiencing unintended perioperative hypothermia (<36°C),20 in surgeries where the PiCCO system is used to hemodynamic monitoring.
The major limitation of this study is the sample size. This fact also precluded the stratification for different CO values which could be useful for more detailed analysis. Larger randomized studies are needed to confirm this inadequacy of PiCCO, and possibly other thermodilution methods, for patients under temperatures <36°C.
Despite the very good results of the echocardiographic preliminary study, as a technique observer-dependent, it must always be considered as a potential source of bias.
Author contributionThe Authors Teresa Souto Moura, Sílvia Aguiar Rosa, Nuno Germano, Raquel Cavaco, Tania Sequeira and Luis Bento, declare that they have made substantial contributions to the conception and design of the study, the acquisition of data and the interpretation of the data.
Marta Alves, Ana Luisa Papoila have performed the analysis and interpretation of the data.
All the authors have commented on the draft of the article and critical revision of the intellectual content, as well as the definitive approval of the version that is presented.
Conflicts of interestThe authors declare that there is no conflict of interest.
The authors wish to thank the medical team of the Unidade de Urgência Médica for their help during the implementation of the study.