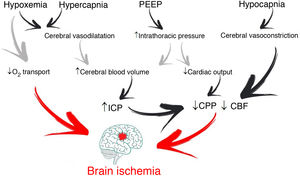
It is common for patients with acute brain injury (ABI) admitted to the Intensive Care Unit (ICU) to require endotracheal intubation and to be exposed to respiratory failure1. Management involves maintaining correct gas exchange and stable brain conditions (intracranial pressure [ICP] <22 mmHg and cerebral perfusion >60 mmHg), with the aim of avoiding secondary hypoxic damage (Fig. 1)2.
Acute respiratory distress syndrome (ARDS) develops in 20% of these patients and is associated with poorer outcomes. This constitutes a great challenge for healthcare teams, since it reflects the differences between the recommendations for the management of patients with ABI and those considered to reflect “best practice” in patients with ARDS3,4.
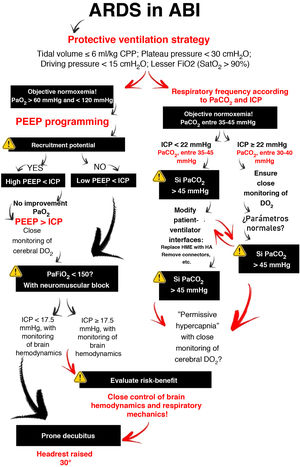
How can we personalize mechanical ventilation in hypoxemic patients with ABI?Concerning mechanical ventilation (MV), strategies in conflict with lung protection are often used in patients with ABI. To reduce the risk of injury, the ventilation strategy should focus on limiting the end-inspiratory pressures through the use of a low tidal volume (Fig. 2). However, this is associated with PaCO2 levels above those recommended for the optimization of cerebral blood flow (CBF).
Through changes in extracellular pH, CO2 modulates cerebrovascular tone, cerebral blood volume and CBF. An increase can generate ICP elevation if brain distensibility is deficient, while a decrease can reduce the cerebral blood volume and CBF through vasoconstriction and give rise to secondary ischemic damage5. Depending on the clinical situation and the ICP of the patient, the recommended PaCO2 values range from 30 to 45 mmHg1. However, if the lung protection strategy prevails, and PaCO2 consequently increases, multimodal brain monitoring (hemoglobin saturation in the bulbar zone of the internal jugular vein >50%, and particularly brain tissue oxygen pressure >15 mmHg), together with systemic oxygenation measurements can allow PaCO2 values higher than those recommended, including even hypercapnia in progressive ranges4.
OxygenationIn the context of ABI, hypoxemia implies greater secondary injury, expressed as ischemia. The Brain Trauma Foundation recommends avoiding PaO2 <60 mmHg2, corresponding to ranges somewhat above those proposed in the case of ARDS3. However, hyperoxia is also associated with poorer outcomes, producing harmful effects as a consequence of excessive reactive oxygen species (ROS) production, with damage at cardiovascular, pulmonary and cerebral level6. On the other hand, PaO2 values within the normoxia range, with the lowest possible FiO2, are recommended, with consideration of the possibility of acting upon those variables that affect the relationship between DO2 and brain oxygen consumption, in favor of transport — if so required by the situation of the patient7.
Positive end-expiratory pressure (PEEP)Positive end-expiratory pressure (PEEP) can affect cerebral circulation via two routes: reduced venous return to the right side of the heart and through CO2-mediated mechanisms. The decrease in mean blood pressure due to increased PEEP and right atrial pressure can reduce CBF (if cerebral self-regulation is altered) or keep it constant and elevate ICP. However, if euvolemia is guaranteed, mean blood pressure and cerebral perfusion pressure (CPP) do not experience significant variations4. The decrease in cerebral venous return depends on the ICP-PEEP relationship and pressure transmission through the cerebral veins, according to the Starling resistor model. Positive end-expiratory pressure increases intrathoracic pressure and the pressure in the right atrium, and thus also the pressure in the sagittal venous sinus, which reduces cerebral venous flow and increases ICP. Accordingly, provided PEEP is lower than ICP, venous return should not be hindered.
On the other hand, PaCO2 resulting from the application of PEEP depends on its effects on gas exchange and respiratory mechanics. Only those patients who in the face of PEEP elevation suffer increased lung elastance will be exposed to higher CO2 levels with significant ICP increments8. Therefore, provided PEEP is lower than ICP, the impact on brain hemodynamics will depend on the effect of PEEP in terms of lung recruitment or overdistension — though it must be noted that no study has clearly established what PEEP levels are harmful to patients with ABI.
What happens if even so the patient with ABI remains hypoxemic?This situation makes it necessary to balance the expected improvements in lung and brain oxygenation against the potentially harmful effects in relation to ICP and cerebral perfusion pressure. The use of neuromuscular blockers is advised, despite the low-level evidence, due to the few risks and the positive impact on mortality9. It should be reserved for patients suffering ARDS with PaFiO2 <150 and in the early stages of the disorder. Alveolar recruitment maneuvers can reduce atelectasis and increase lung volume; however, they are not advised, since there is no consistent evidence justifying such use. The improvements in oxygenation are usually temporary, and the consequences pose high risks in patients with ABI. Prone decubitus is the most controversial intervention due to its impact on survival in ARDS10. Based on the available evidence, the experts strongly recommend prone decubitus in the case of low ICP, though not so in the context of high ICP. The benefits in terms of lung elastance and oxygenation, maintaining adequate cerebral perfusion pressure, may outweigh the risk of increased ICP.
Lastly, the use of extracorporeal membrane oxygenation (ECMO) in patients with ABI historically has been limited by the risk of cerebral hemorrhage related to anticoagulation, and although some recent reports have suggested a possible use of the technique in patients with ABI, the body of evidence remains small. In this context, the possibility of adopting venovenous ECMO without heparin constitutes a field for future research.
ConclusionsMost patients with severe ABI require mechanical ventilation, and of these, many develop lung injuries. Adequate and personalized ventilator management is crucial to avoid secondary lesions due to hypoxemia and hypo- or hypercapnia. Protective ventilation strategies can be safely used in most patients with ABI.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Plotnikow GA, del Bono MR. Lesión cerebral aguda e hipoxemia: individualización del soporte ventilatorio. Med Intensiva. 2022;46:521–523.