Cerebral venous sinus thrombosis (CVST) is a rare entity that often attacks young people (under 50) and represents 0.5%–1% of all strokes reported.1 The coronavirus 2019 (COVID-19) pandemic has had a tremendous impact on society and the healthcare system. To this date, the development of vaccines is the main therapeutic tool available.2 We describe 2 cases of CVST that required admission to the intensive care unit (ICU).
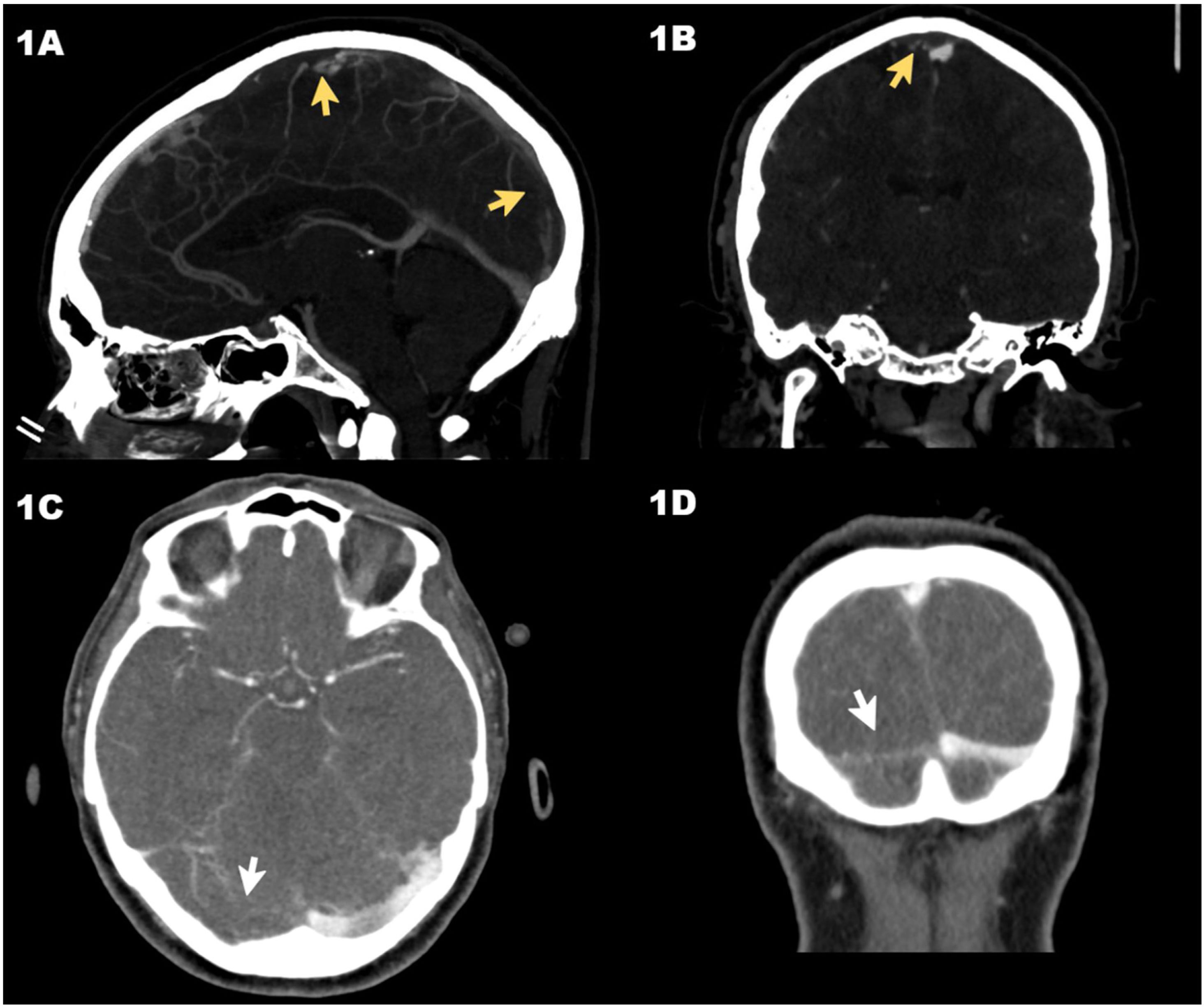
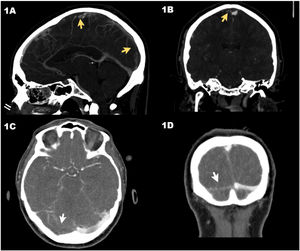
Case #1 is a 30-year-old man who was admitted to the ER with intense headache of 8-day evolution after receiving the first dose of the vaccine of the recombinant adenoviral vector that codifies the antigen of the spike protein of the acute respiratory distress syndrome (SARS-CoV-2) (ChAdOx1 nCov-19, AstraZeneca). There were no findings on the cranial computed tomography (CT) scan performed. After 8 h at the ER, the patient still complained of a headache showing elevated D-dimer levels that went from 5360 ng/mL to 49 732 ng/mL. The cranial CT scan with vascular study was repeated (venogram included) and revealed the presence of a superior longitudinal CVST (Fig. 1A and B). Patient’s disease progressed into thrombocytopenia (78 000 platelets/μL), and eventually the diagnosis of vaccine-induced prothrombotic immune thrombocytopenia (VIPIT) was confirmed testing positive for anti-platelet factor 4 (anti-PF4) antibodies using the enzyme-linked immunosorbent assay (ELISA) technique. The patient developed generalized tonic-clonic seizures with low level of consciousness and a Glasgow coma scale (GCS) of 6 (M: 4, O: 1, V: 1). He was admitted to the ICU with presence of dilated non-reactive pupils. A different cranial CT scan was performed that confirmed the presence of a left parietal acute venous infarction with signs of hemorrhagic transformation and significant cerebral swelling. Together with the Neurosurgery and Neuroradiology Unit, it was decided to implant an external ventricular drainage (EVD) catheter followed by mechanical thrombectomy. Procedures were performed uneventfully and proper angiographic results after 2 passes with the Retriever® stent (Stryker Neurovascular, Kalamazoo, MI, United States) were confirmed on the digital subtraction angiography. The patient developed refractory intracranial hypertension to third level measures. The transcranial Doppler echocardiography revealed the presence of systolic waves. The patient remained on argatroban in doses of 0.5 μg/kg/min to 1 μg/kg/min adjusted for an activated partial thromboplastin time of 1.5 to 3 times with respect to control, and non-specific human immunoglobulins (Flebogamma®; Grifols, S. A., Barcelona, Spain) at doses of 1 g/kg/day for 3 days. Finally, on day 4, the diagnosis of brain dead was confirmed.
Axial computed tomography (CT) scan in venous phase (case #1) showing the thrombosis of the superior longitudinal venous sinus (arrows) in sagittal (A) and coronal (B) views. CT scan in venous phase (case #2) showing the lack of repletion of the right transverse venous sinus (arrows) in the sagittal (C) and coronal (D) views.
Case #2 is a 64-year-old woman admitted to the ER due to headache of 2-week evolution with worsening 48 h prior to admission and walking instability. She had just received the first dose of the ChAdOx1 nCov-19 vaccine. The patient had a good level of consciousness (GCS 15). The cranial CT scan confirmed the presence of a right cerebellar infarction with hemorrhagic transformation while the vascular study revealed the presence of CVST to the right transverse sinus (Fig. 1C and D). The patient had thrombocytopenia (64 240 platelets/μL) and elevated D-dimer levels of up to 51 292 ng/mL. She was then transferred to our center where a possible VIPIT study was conducted that would be later confirmed with positivity for anti-PF4 antibodies. The patient was managed conservatively and kept on neuromonitoring. Anticoagulant therapy with argatroban and Flebogamma® was administered at similar doses compared to case #1. Twelve hours after ICU admission, the patient started showing non-reactive mydriasis in her right pupil and a lowered level of consciousness with an impaired GCS of 8 (M: 4, O: 2, V: 2). The cranial CT scan revealed the presence of hydrocephaly. It was decided to implant an EVD catheter. Disease progression was favorable, and the patient was weaned from mechanical ventilation on day 5 and the EVD catheter removed on day 10. After ICU discharge (day 11), the patient remained conscious, collaborative without neurological focality and a modified Ranking scale score of 1. A summary of the patient’s analysis and clinical characteristics is shown on Table 1.
Summary of the clinical and analytical characteristics, treatments, and results of the 2 cases reported.
| Characteristics | Case #1 | Case #2 |
|---|---|---|
| Age (years) | 30 | 64 |
| Sex | Male | Female |
| Past medical history | None | Hyperactive bladder, osteoporosis |
| Previous treatment | None | Tramadol plus paracetamol, solifenacin, denosumab |
| Vaccine-hospitalization time (days) | 8 | 22 |
| ER-diagnosis time (h) | 18 h 42 min | 1 h 32 min |
| ER-ICU time (h) | 25 h 18 min | 06 h 09 min |
| ER-target anticoagulation time (h) | 33 h 20 min | 22 h 29 min |
| Symptoms | Sudden headache, myalgias | Headache, nausea, vomiting, walking instability |
| Location of the lesions | Thrombosis of superior longitudinal sinus, left parietal infarction with hemorrhagic transformation, cerebral edema | Thrombosis of right transverse venous sinus, cerebellar infarction with hemorrhagic transformation |
| GCS (M, O, V) during ICU assessment | GCS 6 (M: 4, O: 1, V: 1) | GCS 15 (M: 6, O: 4, V: 5) |
| Pupil alterations | Dilated and non-reactive (bilateral) | Non-reactive unilateral mydriasis (right pupil) |
| Seizures | Yes | No |
| Anti-PF4 antibodies | Positive | Positive |
| Anticoagulation | Argatroban | Argatroban |
| Other therapies | Immunoglobulins, EVD catheter, mechanical thrombectomy | Immunoglobulins, EVD catheter |
| Results at ICU discharge | Dead | Alive | ||
|---|---|---|---|---|
| Admission | Nadir (day) | Admission | Nadir (day) | |
| Platelets (100–400 × 1000/μL) | 212 | 9 (3) | 64 | 49 (2) |
| APTT (20–38.0 s) | 25 | 90.2 (3) | 30 | 65.7 (3) |
| TT (15.8 s) | 17 | NA | 19.2 | NA |
| Prothrombin activity (70% to 120%) | 88 | 18 (3) | 87 | 35 (3) |
| INR (0,8–12) | 1.1 | 3.8 (3) | 1,07 | 1.9 (3) |
| Fibrinogen (derived, 180 mg/dL to 350 mg/dL) | 492 | 272 (2) | 229 | 234 (2) |
| D-dimer (0.0 ng/mL to 500.0 ng/mL) | 5360 | 220 914 (2) | 51 292 | 64 805 (2) |
| CRP (0–8.0 mg/dL) | 26.7 | 100.7 (2) | 47.9 | 50.2 (2) |
| SARS-CoV-2 CRP | Negative | Negative | ||
APTT, activated partial thromboplastin time; CRP, C-reactive protein; EVD, external ventricular drainage; GCS, Glasgow Coma Scale; h, hours; ICU, intensive care unit; INR, international normalized ratio; M, motor; mg/dL, milligrams per deciliters; NA, not assessed; ng/mL, nanograms per deciliters; O, ocular; PF4, platelet factor 4; s, seconds; SARS-CoV-2 RCP, polymerase chain reaction of severe acute respiratory distress syndrome coronavirus 2; TT, thrombin time; U/L, units per liter; V, verbal.
CVST is a rare entity with an annual incidence rate between 0.22 and 1.57 for every 100 000 inhabitants. It mostly affects young women (35 to 40 years) and is associated with genetic or acquired thrombotic states.1
It has been confirmed that SARS-CoV-2 infection is a risk factor for developing CVST. A retrospective study showed a higher rate of CVST after the beginning of the COVID-19 pandemic (42.8 per million of inhabitants), which is higher compared to patients with influenza (RR, 3.83, 95%CI, 1.56-9.41, P < .001), and people who received the mRNA vaccine (RR, 6.67, 95%CI, 1.98-22.43, P < .001).3
Recently, episodes of thrombosis and thrombocytopenia have been associated with the use of certain vaccines against SARS-CoV-2 (the so-called VIPIT). Seemingly, its etiopathogenesis is due to an autoimmune mechanism similar to the one reported in heparin-induced thrombocytopenia.4 Its management and diagnosis are not fully understood. However, a high mortality rate, the appearance of thrombosis in unusual sites, and the presence of anti-PF4 antibodies have been reported in heparin-naïve patients.4,5
After the appearance of the first few cases of CVST in Spain, the use of the ChAdOx1 nCov-19 vaccine6 was suspended in this country. By the time the second hospitalization was reported, Spain had already administered 3,817,930 doses of the ChAdOx1 nCov-19 vaccine, out of which 161 800 had already been administered in our region (Castile-La Mancha).7 According to the 5th report on pharmacovigilance published by the Spanish Agency of Medicines and Medical (AEMPS), 11 cases of VIPIT had already been reported in Spain, 3 of which resulted in death.8 We reported on 2 cases of TSVC associated with the ChAdOx1 nCov-19 vaccine, 1 of which resulted in death. The management of this entity followed the international recommendations with immunoglobulins and anticoagulant therapy with argatroban. We should mention that fondaparinux, bivalirudin, and direct factor Xa inhibitors like rivaroxaban and apixaban are feasible alternatives to argatroban.9
The heterogeneity reported in the clinical presentation complicates its early approach, which can have an impact on results.5 The patient who died had longer times since he was admitted to the ER until diagnosis was achieved (18 h 42 min vs 1 h 32 min), until ICU admission (25 h 18 min vs 6 h 09 min), and until target anticoagulation was reached (33 h 20 min vs 22 h 29 min). Mechanical thrombectomy was not useful either despite the good angiographic result reported.
To this date, CVST is an extremely rare entity whose association with the vaccine has been cause for alarm due to its severity and the impact that suspending the administration of the vaccine would have under the present circumstances. However, many aspects still need to be elucidated regarding origin, presentation, disease progression, prognosis, and management. We should mention here that a normal CT scan does not exclude the diagnosis of CVST. In any case, accelerating the diagnostic and therapeutic approach can impact the results, which is why it is important to pay attention to patients who, after vaccinated, present with compatible clinical signs.
We wish to thank the medical personnel of the Intensive Care Unit at Complejo Hospitalario de Toledo, Spain.
Please cite this article as: Morales Varas G, Calle Flores A, Sánchez Casado M. Trombosis de senos venosos tras vacunación con ChAdOx1 nCov-19. Med Intensiva. 2022;46:524–527.