Acute respiratory distress syndrome (ARDS) is currently one of the most important critical conditions, due to its high incidence, mortality rate, long-term sequelae and nonspecific pharmacological treatment.
The histological hallmark of ARDS is diffuse alveolar damage (DAD). Approximately 50% of all ARDS patients present DAD, while the rest exhibit a heterogeneous group of histological patterns, many of which correspond to well-recognized disease. Consequently, if these patterns could be diagnosed, patients could benefit from specific treatment.
The effect of DAD upon the clinical and analytical evolution of ARDS has recently been demonstrated; the classical approach to ARDS as an entity defined solely by clinical, radiological and gasometrical variables therefore should be reconsidered.
This review aims to examine the need to evolve from the concept of ARDS as a syndrome to ARDS as a specific disease. Accordingly, we have addressed four critical questions: (a) what is a disease?; (b) what is DAD?; (c) how do the different definitions of ARDS regard DAD?; and (d) what is the relationship between ARDS and DAD?
El síndrome de distrés respiratorio agudo (SDRA) constituye una de las entidades más importantes de la medicina crítica dada su elevada incidencia, mortalidad, secuelas a largo plazo y ausencia de un tratamiento farmacológico específico.
El patrón histológico característico del SDRA es el daño alveolar difuso (DAD). Aproximadamente el 50% de los pacientes con SDRA tienen DAD; el resto está constituido por un grupo heterogéneo de patrones histológicos, muchos de los cuales constituyen enfermedades bien caracterizadas que, de ser diagnosticadas, podrían beneficiarse de un tratamiento específico.
Recientemente se ha demostrado el efecto del DAD en la evolución clínica y analítica del SDRA, por lo cual, el enfoque clásico del SDRA como una entidad definida exclusivamente por variables clínicas, radiológicas y gasométricas podría ser reconsiderado.
La presente revisión narrativa procura analizar la necesidad de evolucionar desde el concepto de SDRA como síndrome a SDRA como enfermedad; para ello hemos planteado 4 preguntas que consideramos prioritarias: a) ¿qué es una enfermedad?; b) ¿qué es el DAD?; c) ¿cómo consideran al DAD las distintas definiciones de SDRA?, y d) ¿qué relación existe entre el DAD y el SDRA?
Despite the technological advances, investigations and professional training, acute respiratory distress syndrome (ARDS) remains a very important condition in Intensive Care Medicine, with an incidence of between 7.2 and 86.2 cases per 100,000 patients-year,1–9 and a mortality rate of between 32 and 61%.2–8,10–12 In recent years we have witnessed substantial advances in the optimization of ventilatory support13–17; however, the development of drug treatment specific for ARDS remains a major challenge.18–20
A relationship between ARDS and diffuse alveolar damage (DAD) was postulated from the first description of ARDS almost 50 years ago.21 However, only about 50% of all patients with ARDS have DAD; the rest of cases comprise a heterogeneous series of histological patterns, many of which correspond to well characterized disease conditions. Consequently, if such patterns could be adequately diagnosed, patients could benefit from the prescription of specific treatment. As an example, postmortem studies have shown that 6.5% of the patients clinically diagnosed with ARDS in fact presented tuberculosis, pulmonary thromboembolism or neoplastic infiltration, and that in 7.5% of the patients the lungs were normal. These latter cases could correspond to atelectasis, since lung tissue fixation requires insufflation of the organ with formaldehyde, which usually causes atelectasis to disappear, leaving the lungs with an apparently healthy histological appearance.22 Furthermore, the recent demonstration of an association between DAD and ARDS outcome (vide infra) could imply that the classical approach to ARDS as a condition exclusively defined on the basis of clinical, radiological and gasometric variables should be reconsidered.
The present review aims to analyze the need to evolve from the concept of ARDS as a syndrome to ARDS as a disease. In this regard, we have addressed four questions which we regard as fundamental: (a) what is a disease?; (b) what is DAD?; (c) how do the different definitions of ARDS regard DAD?; and (d) what is the relationship between DAD and ARDS?
What is a disease?Diagnostic algorithms are the tools used by clinicians to rationally advance from the symptoms and signs causing the patient to seek medical help (usually grouped as syndromes) to the diagnosis of a specific disease that may benefit from guided treatment.23 “Disease” is possibly one of the most commonly used words in Medicine, and although the term is very familiar to us, its definition is a great challenge.24
From the semantic perspective, the dictionary of the Spanish National Royal Academy of Medicine defines disease as a “series of alterations, signs and symptoms that organize according to a concrete scheme in time and space in response to a concrete cause, and which manifest in a similar way in different individuals, thus allowing classification and identification of the different disorders”.25
From the medical and clinical epidemiological perspective, the relevant events or variables are only those which: (a) affect mortality (death); (b) allow the diagnosis of a disease; (c) have an economical impact (destitution); or (d) cause discomfort; (e) disability; and/or (f) dissatisfaction in the patient (the so-called “6Ds”: death, disease, destitution, discomfort, disability, dissatisfaction).26 Thus, from this perspective, a disease is defined as a series of signs and symptoms implying similar 6Ds. Evidently, there may be certain differences leading to the identification of different subtypes, classes or endophenotypes, but these cannot condition substantial variations in the 6Ds. As an example, up until only a few years ago a cancer could be classified simply on the basis of the histology and TNM classification. At present this approach is clearly insufficient, and the genetic, epigenetic and proteomic differences also must be addressed in order to subclassify most cancers and thus optimize the diagnosis, prognosis and treatment.27,28 The magnitude of the difference between two conditions required in order to consider them to be two different diseases or subtypes of one same disease remains to be established, though it undoubtedly will be based on the combination of clinical (6Ds), histological (e.g., light microscopy, confocal microscopy, etc.) and molecular factors (e.g., genomics, proteomics, metabolomics, etc.).
What is diffuse alveolar damage (DAD)?In 1935, Louis Hamman and Arnold Rich described a histological pattern (which later became known as the organizing phase of DAD) in four patients who died due to hypoxemia of unknown cause, characterized by the diffuse interstitial proliferation of fibroblasts.29,30 In 1986, Katzenstein et al.,31 with the purpose of reducing the confusion between the clinical course and the histological findings, proposed two terms: (a) acute interstitial pneumonia (AIP) referred to patients with an acute course and DAD (previously referred to as Hamman–Rich syndrome); and (b) usual interstitial pneumonia or idiopathic pulmonary fibrosis referred to patients with a chronic course and DAD.31,32
The consideration of ARDS adds a new confounding factor, since DAD is a diagnostic criterion of acute interstitial pneumonia, but also a hallmark of ARDS.33 In other words, if the pathologist observes findings consistent with DAD (Fig. 1) and the patient does not meet the clinical criteria of ARDS, the diagnosis should be acute interstitial pneumonia or usual interstitial pneumonia. In contrast, in the presence of clinical criteria of ARDS, the diagnosis should be ARDS with DAD.
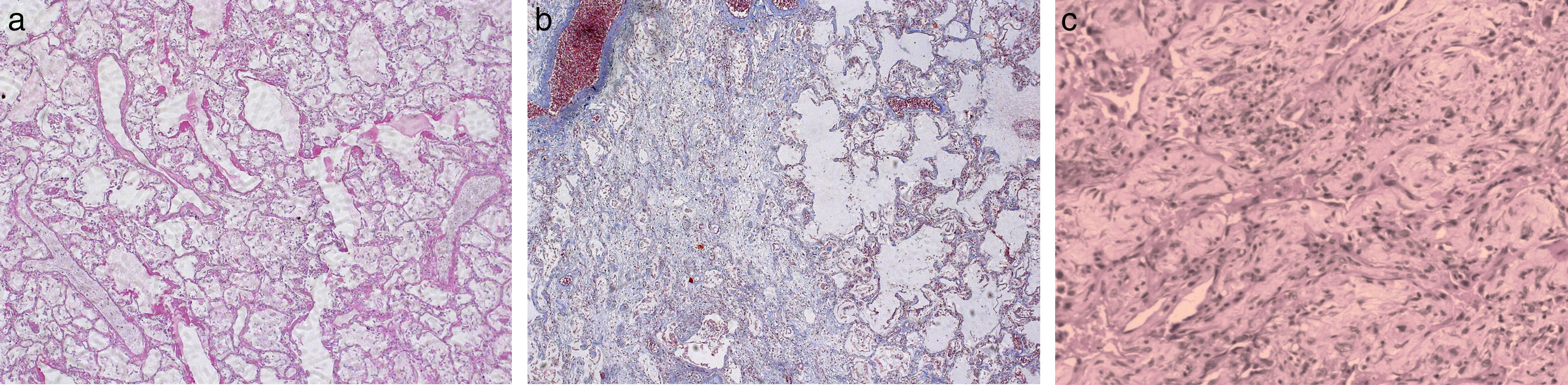
Diffuse alveolar damage (DAD). Case 1: A 42-year-old patient admitted to the intensive care unit (ICU) with septic shock associated to acute nosocomial pneumonia. Death occurred due to refractory hypoxemia after two days on mechanical ventilation. The postmortem study (Fig. a, hematoxylin–eosin [HE], ×5) showed a heterogeneous histological pattern with areas of DAD in the exudative phase, with the presence of hyaline membranes and interstitial and alveolar edema, vascular congestion of the interalveolar septae, and desquamation and hyperplasia of the type II pneumocytes. In other areas (Fig. b, HE, ×5) DAD in the organizing phase with lax interstitial and intraalveolar fibrosis was noted, together with hyaline membranes and the rest of the previously mentioned findings. Case 2: A 28-year-old patient admitted to the ICU due to ARDS secondary to respiratory sepsis attributed to H1N1 influenza. Death occurred due to refractory hypoxemia after 48 days on mechanical ventilation. The lung tissue micrograph (Fig. c, HE, ×5) obtained at autopsy showed evolved DAD in the fibrotic stage, with great distortion of the lung parenchyma and practically complete substitution of the alveolar spaces with fibrotic tissue.
According to Katzenstein et al.,34 DAD is a specific reaction of the lungs to a range of aggressive factors. The common feature is endothelial and alveolar damage giving rise to the exudation of fluids and cells, with progression in some cases to extensive interstitial lung fibrosis. The most characteristic pathological finding of the acute phase of DAD is the presence of hyaline membranes, which under the light microscope appear as a homogeneous and eosinophilic material extending over the internal surface of the alveoli (Fig. 1a and b). These hyaline membranes are composed of the necrotic remains of epithelial cells and serum proteins that have penetrated from the bloodstream into the alveolar space secondary to an increase in permeability of the alveolar-capillary barrier. One of the most widely debated issues, and for which no firm answers have been obtained, is the need or not to include the presence of hyaline membranes in the diagnosis of DAD. At present, the studies involving the largest number of patients, as well as the only study demonstrating evolutive differences between patients with ARDS according to the presence or absence of DAD, have considered the presence of hyaline membranes to be essential for the diagnosis of DAD.22,35–38
The morphological features of DAD depend on the time elapsed from risk factor exposure to the moment of the histological study (Fig. 1).34,36,39 Schematically, three consecutive phases can be observed: an early or exudative phase (Fig. 1a) characterized by an intraalveolar exudate, hyaline membranes and a cellular infiltrate mainly composed of lymphocytes, plasma cells and macrophages; an intermediate or proliferative phase characterized by hyperplasia, atypia and mitosis of type II pneumocytes, with thrombosis of the small pulmonary arteries; and a final fibrotic or organizing phase (Fig. 1b) characterized by thickening of the alveolar-capillary membrane, the proliferation of fibroblasts particularly at interstitial level, and fibrosis. In the more severe cases, fibrosis can progress over weeks, restructuring the entire lung parenchyma and generating a characteristic “honeycomb lung” image, with practically total substitution of the alveolar space (Fig. 1c). It is important to mention that the different phases can overlap; as a result, different evolutive phases can be found in one same patient (Fig. 1).36
How do the different definitions of ARDS regard diffuse alveolar damage?The evolution of the concept of ARDS since the classical term idiopathic pulmonary anasarca postulated by Laënnec in the year 1821 to the recent definition of Berlin33,40 has had a considerable impact on the designing of research and on the measurement of the variables implicated in the 6Ds.40,41 Of the many proposed definitions,21,33,42–48 only 5 have received the necessary acceptance to be regarded as genuine definitions of ARDS.21,33,42–44
The first definition of ARDS was proposed by Ashbaugh et al.21 in the year 1967, and although the lung findings in their 7 postmortem studies were described, no histological pattern was included in the definition.
The second definition of ARDS was proposed by Murray et al.44 in the year 1988, and considered three aspects: (a) the chronology of the event (acute or chronic); (b) a composite severity scale (chest X-rays, PaO2/FiO2 ratio, PEEP and lung compliance); and (c) the origin of the lung damage (caused by aspiration pneumonia, lipid embolism, drug use, inhalation of toxic agents or infection; or associated to sepsis, multiple blood transfusions, acute pancreatitis or disseminated intravascular coagulation). This definition likewise contemplated no histological pattern.
The third definition of ARDS was the result of two meetings (15 May 1992 in Miami, USA, and 25 October 1992 in Barcelona, Spain) auspiced by the American Thoracic Society and the European Society of Intensive Care Medicine.42 This definition considers ARDS (it includes the term acute lung injury for patients meeting the criteria of ARDS but with a PaO2/FiO2 ratio between 200 and 300) to be an inflammatory syndrome with increased lung permeability, associated to a range of clinical, radiological and physiological anomalies that cannot be explained, but which may coexist with increased left atrial pressure or pulmonary capillary hypertension. The definition also mentions that the lung histological findings may include damage to the endothelial and/or epithelial barrier, as well as a humoral and cellular inflammatory response. However, no mention is made of DAD, and no histological criterion of any kind is included in the definition.
The fourth definition of ARDS was proposed by Ferguson et al.43 in the year 2005, using the Delphi method. Although the general criteria submitted to the participants before the start of the study mentioned that ARDS is the “reflection” of DAD, neither the rest of the manuscript nor the final criteria made any reference to DAD.
The last definition of ARDS is called the Berlin definition, in acknowledgment of the city where the 2012 consensus meeting was held. In the preliminary version of the definition, the panel of experts agreed that ARDS is an acute, diffuse inflammatory type of lung lesion characterized by an increase in vascular permeability and the lose of lung aeration. The clinical hallmarks are hypoxemia and bilateral opacities associated to an increase in pulmonary shunting and physiological dead space. The characteristic morphological finding of the acute phase is composite DAD, e.g., with edema, inflammation, hyaline membranes or hemorrhage. However, the final version of the definition includes no reference to DAD, on the grounds that there is a poor correlation between ARDS and the finding of DAD in postmortem studies.49 In addition, it was underscored that not all the members of the panel of experts agreed with the idea of DAD as the only histological correlate of ARDS, and considered that pneumonia and non-cardiogenic lung edema could also be implicated.50
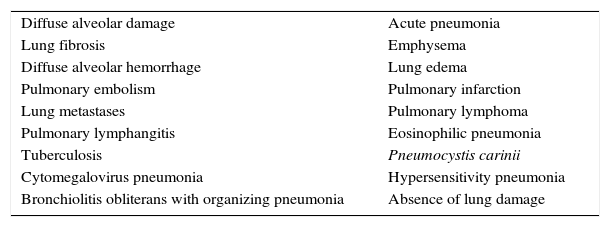
What is the relationship between diffuse alveolar damage and ARDS?Many authors consider DAD to be the most characteristic histological feature of ARDS.21,33,34,43,44,51,52 However, none of the definitions regard DAD as a diagnostic criterion (vide supra). This could be due to two reasons. Firstly, the histological picture that can be found in patients with ARDS is very heterogeneous. Diffuse alveolar damage is observed in no more than 62% of all patients with ARDS22; in the rest of the cases a broad range of histological patterns can be found (Table 1) – many of which are amenable to specific treatment, and which are hard to regard as ARDS (e.g., cancer, pulmonary thromboembolism, absence of histological lesions, etc.). Secondly, in the real world it is practically impossible to obtain direct and unbiased evidence of the effect of DAD upon the 6Ds of ARDS. Conducting a study including a large number of ARDS patients (with different ages, etiologies, degrees of severity, etc.), with histological analysis allowing us to establish the DAD subgroup, would not be possible due to ethical reasons. Consequently, the evidence of the impact of DAD comes from postmortem studies21,22,34–36,53,54 or from highly selected groups of patients with ARDS in which lung biopsies are performed.38,55
The most common histological patterns in patients diagnosed with acute respiratory distress syndrome.
| Diffuse alveolar damage | Acute pneumonia |
| Lung fibrosis | Emphysema |
| Diffuse alveolar hemorrhage | Lung edema |
| Pulmonary embolism | Pulmonary infarction |
| Lung metastases | Pulmonary lymphoma |
| Pulmonary lymphangitis | Eosinophilic pneumonia |
| Tuberculosis | Pneumocystis carinii |
| Cytomegalovirus pneumonia | Hypersensitivity pneumonia |
| Bronchiolitis obliterans with organizing pneumonia | Absence of lung damage |
Despite the above, there is indirect evidence that DAD might be a diagnostic criterion of ARDS. The elegant study published by Calfee et al.,56 using latent class analysis (a strategy aimed at identifying groupings between variables, postulating the existence of subgroups that are latent or not directly observable), showed that the number of endophenotypes (also called latent classes) explaining the distribution of the variables in two cohorts of patients with ARDS is limited to two (other models with 3, 4 and 5 latent classes exhibited poorer performance).57–60 In the derivation cohort, called ARMA and composed of the subgroup of patients with ARDS subjected to low volume ventilation coming from three studies (n=473),57–59 the two endophenotypes were found to be distinguished by clinical (gender, race, need for vasopressors, heart rate, minute-ventilation, arterial pressure), laboratory test (IL-6, IL-8, sTNFr-1, ICAM-1, PAI-1, bicarbonate and protein C) and evolutive characteristics (days of mechanical ventilation and days without multiorgan dysfunction). Similar results were obtained in the validation cohort, called ALVEOLI and composed of patients with ARDS subjected to low volume ventilation coming from a single study (n=549)60 (the best model was that comprising two endophenotypes distinguished according to race, need for vasopressors, heart rate, minute-ventilation, systolic blood pressure, IL-6, IL-8, sTNFr-1, ICAM-1, PAI-1, bicarbonate and protein C, days of mechanical ventilation, days without multiorgan dysfunction and mortality). From the therapeutic perspective, in the ALVEOLI cohort (the design did not allow analysis of the ARMA cohort) it was shown that the response to treatment depends on the endophenotype, since the application of high PEEP in subgroup 1 increased the mortality rate (15% with low PEEP versus 24% with high PEEP), while in subgroup 2 the mortality rate decreased (51% with low PEEP versus 42% with high PEEP).60
Recently, Lorente et al.37 analyzed a group of 149 autopsies of patients with ARDS, and for the first time demonstrated that the presence of DAD is associated to clinical and laboratory test evolution different from that seen in the rest of patients (age, Sequential Organ Failure Assessment [SOFA] score, PaO2/FiO2 ratio, lung compliance, international normalized ratio [INR] and cause of death). They also developed a predictive model for DAD, based on three variables (age, PaO2/FiO2 and lung compliance) measured at the time of diagnosis of ARDS, and resulting in a discriminative capacity as reflected by the area under the receiver operating characteristic (ROC) curve of 0.74 (in the validation cohort the AUC was 0.73). The “inflamed lung” concept was suggested with the purpose of improving the correlation between the clinical and histological observations, and which could include DAD and pneumonia – in which case proportional finding of the characteristic histological feature of the patients with ARDS could reach 88%.50 The finding of clinical and laboratory test differences between patients with ARDS and histological pneumonia versus patients with ARDS and DAD by Lorente et al.37 offers an argument against the above postulate. The added fact of not having found differences in severity, as reflected by the SAPS II score on the day of diagnosis of ARDS between ARDS patients without DAD and with DAD, offers a second argument against the postulate that both histological patterns constitute the same disease condition or two different severity levels of the same disease. Following this same criterion, other conditions such as increased permeability of the alveolar-capillary membrane or apoptosis, should demonstrate their association to DAD in order to be considered characteristic findings of ARDS.
Lastly, Kao et al.,38 in a cohort of 101 patients with ARDS (56 with DAD), found the factors associated to mortality to be the presence of DAD in the lung biopsy (OR 3.554; 95%CI 1.305–9.120; p<0.008) and the sequential organ failure assessment score (OR 1.424; 95%CI 1.187–1.707; p<0.001).
In sum, the advances in modern Medicine have been based on the optimization of the diagnosis, prognosis and treatment. Medical reasoning extends from the general (syndromes) to the concrete (diseases). In this regard ARDS is a syndrome, i.e., a series of signs and symptoms which the physician must observe in order to guide his or her reasoning and ultimately adopt a series of measures to establish the diagnosis of a specific disease.
For decades we have been aware that the correlation between ARDS and DAD is moderate, and that many diseases (which if diagnosed would be amenable to specific treatment) are able to simulate ARDS.
The relevance of DAD in ARDS has been revitalized as a consequence of the recent publications demonstrating the clinical and evolutive differences between patients with ARDS and DAD versus the rest of patients, including those presenting ARDS with pneumonia.
On the basis of all the above, we consider that including DAD as a diagnostic criterion of ARDS could contribute to reduce the variability in 6Ds found in ARDS, and could help us advance toward the identification of a causal relationship between the histological and clinical pictures, with the ultimate aim of characterizing disease due to acute pulmonary distress.
Since lung biopsies are invasive and imply relative risk, it is essential to stimulate clinical translational research for the development of surrogate image or molecular biomarkers of DAD in patients with ARDS.61
Financial supportThe present study received no type of financial support.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Cardinal-Fernández P, Correger E, Villanueva J, Rios F. Distrés respiratorio agudo: del síndrome a la enfermedad. Med Intensiva. 2016;40:168–174.



![Diffuse alveolar damage (DAD). Case 1: A 42-year-old patient admitted to the intensive care unit (ICU) with septic shock associated to acute nosocomial pneumonia. Death occurred due to refractory hypoxemia after two days on mechanical ventilation. The postmortem study (Fig. a, hematoxylin–eosin [HE], ×5) showed a heterogeneous histological pattern with areas of DAD in the exudative phase, with the presence of hyaline membranes and interstitial and alveolar edema, vascular congestion of the interalveolar septae, and desquamation and hyperplasia of the type II pneumocytes. In other areas (Fig. b, HE, ×5) DAD in the organizing phase with lax interstitial and intraalveolar fibrosis was noted, together with hyaline membranes and the rest of the previously mentioned findings. Case 2: A 28-year-old patient admitted to the ICU due to ARDS secondary to respiratory sepsis attributed to H1N1 influenza. Death occurred due to refractory hypoxemia after 48 days on mechanical ventilation. The lung tissue micrograph (Fig. c, HE, ×5) obtained at autopsy showed evolved DAD in the fibrotic stage, with great distortion of the lung parenchyma and practically complete substitution of the alveolar spaces with fibrotic tissue. Diffuse alveolar damage (DAD). Case 1: A 42-year-old patient admitted to the intensive care unit (ICU) with septic shock associated to acute nosocomial pneumonia. Death occurred due to refractory hypoxemia after two days on mechanical ventilation. The postmortem study (Fig. a, hematoxylin–eosin [HE], ×5) showed a heterogeneous histological pattern with areas of DAD in the exudative phase, with the presence of hyaline membranes and interstitial and alveolar edema, vascular congestion of the interalveolar septae, and desquamation and hyperplasia of the type II pneumocytes. In other areas (Fig. b, HE, ×5) DAD in the organizing phase with lax interstitial and intraalveolar fibrosis was noted, together with hyaline membranes and the rest of the previously mentioned findings. Case 2: A 28-year-old patient admitted to the ICU due to ARDS secondary to respiratory sepsis attributed to H1N1 influenza. Death occurred due to refractory hypoxemia after 48 days on mechanical ventilation. The lung tissue micrograph (Fig. c, HE, ×5) obtained at autopsy showed evolved DAD in the fibrotic stage, with great distortion of the lung parenchyma and practically complete substitution of the alveolar spaces with fibrotic tissue.](https://static.elsevier.es/multimedia/21735727/0000004000000003/v2_201703300141/S2173572716000187/v2_201703300141/en/main.assets/thumbnail/gr1.jpeg?xkr=1dZuESKpnCAWr3yCSGZ24A==)


