When addressing the limitations raised by Rodríguez et al.1 in their recent study published in Medicina Intensiva, it is highlighted that, due to the particularities of the database developed during the pandemic, records on relevant aspects of pulmonary mechanics that could be associated with the clinical outcomes of patients were not included. We agree with the authors that such characteristics of pulmonary mechanics in patients undergoing mechanical ventilation can vary significantly depending on the presence or absence of obesity.2,3
Certainly, protective ventilation is based on the administration of tidal volume adjusted to the ideal body weight (IBW) as an essential part of its approach. However, estimating IBW poses significant challenges: for example, English origin of the formulas used, consistent with an old rule developed from height and weight tables lacks consideration of the patient’s age, which can lead to inaccuracies when applied to populations different from those used in its formulation.4
To offer a more complete perspective in this regard and under the hypothesis that in obese patients undergoing mechanical ventilation, the energy load parameters will vary considerably from one individual to the next, as opposed to non-obese patients, and that this variability will be closely associated with the degree of hypoxemia experienced, we present our pulmonary mechanics data in patients with C-ARDS, categorized by degree of hypoxemia and obesity.
This is a retrospective, observational, and analytical cohort study of all cases hospitalized due to SARS-CoV-2 infection with ICU admission from March 2020 through March 2022. Data were obtained from the COVID-19 patient cohort registry of an intensive care unit in a tertiary referral center. The local Research Ethics Committee approved the study, and informed consent (written and/or via phone call) was obtained from the patients/legal representatives.
During the analyzed period, a total of 911 patients were admitted to the ICU with SARS-CoV-2 disease. After excluding patients younger than 18 years, those who were ventilated in pressure-controlled mode, and those with defective or incomplete records, data analysis was conducted on a total of 253 patients.
Patients were categorized as severely hypoxemic or non-severely hypoxemic based on the value of the arterial partial pressure of oxygen to the fraction of inspired oxygen (P/F) ratio at ICU admission. P/F values <150 mmHg were categorized as severely hypoxemic, while P/F values ≥ 150 mmHg were categorized as non-severely hypoxemic. Based on body mass index (BMI), patients were categorized upon ICU admission as obese with BMI ≥ 30 kg/m2, or non-obese with BMI < 30 kg/m2.5 For analysis, patients were categorized into 4 groups: group #1: patients without severe hypoxemia or obesity; group #2: patients with severe hypoxemia without obesity; group #3: patients without severe hypoxemia with obesity; group #4: patients with severe hypoxemia and obesity.
In calculating bioenergetic variables, Mechanical Power (MP) was defined based on Gattinoni’s simplified formula, Driving Power as: VT × f × [(Pplateau − PEEP)/2] and Dynamic Power as: VT × f × [(Pplateau + PEEP)/2].3,6–8
Inter-group comparisons of percentages were made using ANOVA, and continuous variables were compared using the Kruskal–Wallis test. A multivariate logistic regression analysis was used to explore the association of variables with the primary outcome: 28-day mortality, for each risk factor considered in 3 different models: MP Model, Driving Power Model, and Dynamic Power Model. To interpret the results in a valid clinical scenario, covariates that showed significant differences in the bivariate analysis or a trend (p < 0.2) without evidence of multicollinearity issues (assessed with a Variance Inflation Factor (VIF) <3) were included. The analyzed models are presented as odds ratios (OR) with their 95% confidence intervals (95%CI).
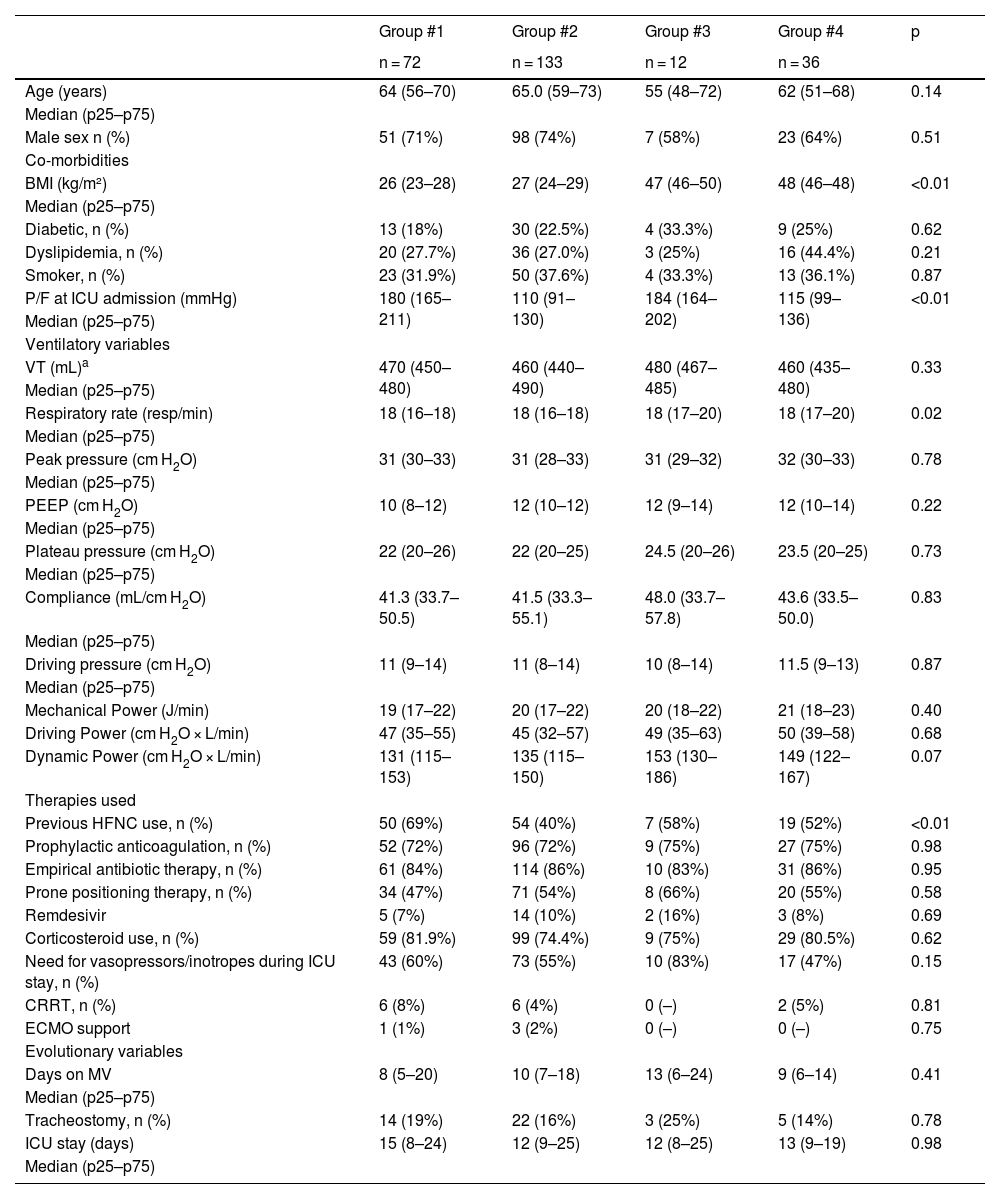
Group #4 (patients with severe hypoxia and obesity) had the highest average MP values: 20.96 J/min. No significant differences were found in the inter-group analysis. The highest mean Driving Power value was evidenced in group #4 (patients with severe hypoxia and obesity): 49.91 cm H2O × L/min; while the highest mean Dynamic Power value was observed in group #3 (patients without severe hypoxemia with obesity): 153.13 (129.75–185.95) cm H2O × L/min (Table 1). Dynamic Power showed significant differences between patient groups, considering the presence of the obesity variable in the group categorization.
Clinical-epidemiological characteristics and mechanical ventilation data.
| Group #1 | Group #2 | Group #3 | Group #4 | p | |
|---|---|---|---|---|---|
| n = 72 | n = 133 | n = 12 | n = 36 | ||
| Age (years) | 64 (56–70) | 65.0 (59–73) | 55 (48–72) | 62 (51–68) | 0.14 |
| Median (p25–p75) | |||||
| Male sex n (%) | 51 (71%) | 98 (74%) | 7 (58%) | 23 (64%) | 0.51 |
| Co-morbidities | |||||
| BMI (kg/m²) | 26 (23–28) | 27 (24–29) | 47 (46–50) | 48 (46–48) | <0.01 |
| Median (p25–p75) | |||||
| Diabetic, n (%) | 13 (18%) | 30 (22.5%) | 4 (33.3%) | 9 (25%) | 0.62 |
| Dyslipidemia, n (%) | 20 (27.7%) | 36 (27.0%) | 3 (25%) | 16 (44.4%) | 0.21 |
| Smoker, n (%) | 23 (31.9%) | 50 (37.6%) | 4 (33.3%) | 13 (36.1%) | 0.87 |
| P/F at ICU admission (mmHg) | 180 (165–211) | 110 (91–130) | 184 (164–202) | 115 (99–136) | <0.01 |
| Median (p25–p75) | |||||
| Ventilatory variables | |||||
| VT (mL)a | 470 (450–480) | 460 (440–490) | 480 (467–485) | 460 (435–480) | 0.33 |
| Median (p25–p75) | |||||
| Respiratory rate (resp/min) | 18 (16–18) | 18 (16–18) | 18 (17–20) | 18 (17–20) | 0.02 |
| Median (p25–p75) | |||||
| Peak pressure (cm H2O) | 31 (30–33) | 31 (28–33) | 31 (29–32) | 32 (30–33) | 0.78 |
| Median (p25–p75) | |||||
| PEEP (cm H2O) | 10 (8–12) | 12 (10–12) | 12 (9–14) | 12 (10–14) | 0.22 |
| Median (p25–p75) | |||||
| Plateau pressure (cm H2O) | 22 (20–26) | 22 (20–25) | 24.5 (20–26) | 23.5 (20–25) | 0.73 |
| Median (p25–p75) | |||||
| Compliance (mL/cm H2O) | 41.3 (33.7–50.5) | 41.5 (33.3–55.1) | 48.0 (33.7–57.8) | 43.6 (33.5–50.0) | 0.83 |
| Median (p25–p75) | |||||
| Driving pressure (cm H2O) | 11 (9–14) | 11 (8–14) | 10 (8–14) | 11.5 (9–13) | 0.87 |
| Median (p25–p75) | |||||
| Mechanical Power (J/min) | 19 (17–22) | 20 (17–22) | 20 (18–22) | 21 (18–23) | 0.40 |
| Driving Power (cm H2O × L/min) | 47 (35–55) | 45 (32–57) | 49 (35–63) | 50 (39–58) | 0.68 |
| Dynamic Power (cm H2O × L/min) | 131 (115–153) | 135 (115–150) | 153 (130–186) | 149 (122–167) | 0.07 |
| Therapies used | |||||
| Previous HFNC use, n (%) | 50 (69%) | 54 (40%) | 7 (58%) | 19 (52%) | <0.01 |
| Prophylactic anticoagulation, n (%) | 52 (72%) | 96 (72%) | 9 (75%) | 27 (75%) | 0.98 |
| Empirical antibiotic therapy, n (%) | 61 (84%) | 114 (86%) | 10 (83%) | 31 (86%) | 0.95 |
| Prone positioning therapy, n (%) | 34 (47%) | 71 (54%) | 8 (66%) | 20 (55%) | 0.58 |
| Remdesivir | 5 (7%) | 14 (10%) | 2 (16%) | 3 (8%) | 0.69 |
| Corticosteroid use, n (%) | 59 (81.9%) | 99 (74.4%) | 9 (75%) | 29 (80.5%) | 0.62 |
| Need for vasopressors/inotropes during ICU stay, n (%) | 43 (60%) | 73 (55%) | 10 (83%) | 17 (47%) | 0.15 |
| CRRT, n (%) | 6 (8%) | 6 (4%) | 0 (–) | 2 (5%) | 0.81 |
| ECMO support | 1 (1%) | 3 (2%) | 0 (–) | 0 (–) | 0.75 |
| Evolutionary variables | |||||
| Days on MV | 8 (5–20) | 10 (7–18) | 13 (6–24) | 9 (6–14) | 0.41 |
| Median (p25–p75) | |||||
| Tracheostomy, n (%) | 14 (19%) | 22 (16%) | 3 (25%) | 5 (14%) | 0.78 |
| ICU stay (days) | 15 (8–24) | 12 (9–25) | 12 (8–25) | 13 (9–19) | 0.98 |
| Median (p25–p75) | |||||
Group #1: non-obese and non-hypoxemic patients; group #2: hypoxemic and non-obese patients; group #3: obese and non-hypoxemic patients; group #4: obese and hypoxemic patients. BMI, body mass index; CRRT, continuous renal replacement therapies; ECMO, extracorporeal membrane oxygenation; HFNC, high-flow nasal cannula; P/F, ratio of arterial partial pressure of oxygen to the fraction of inspired oxygen; PEEP, positive end-expiratory pressure; RR, respiratory rate; VT, tidal volume.
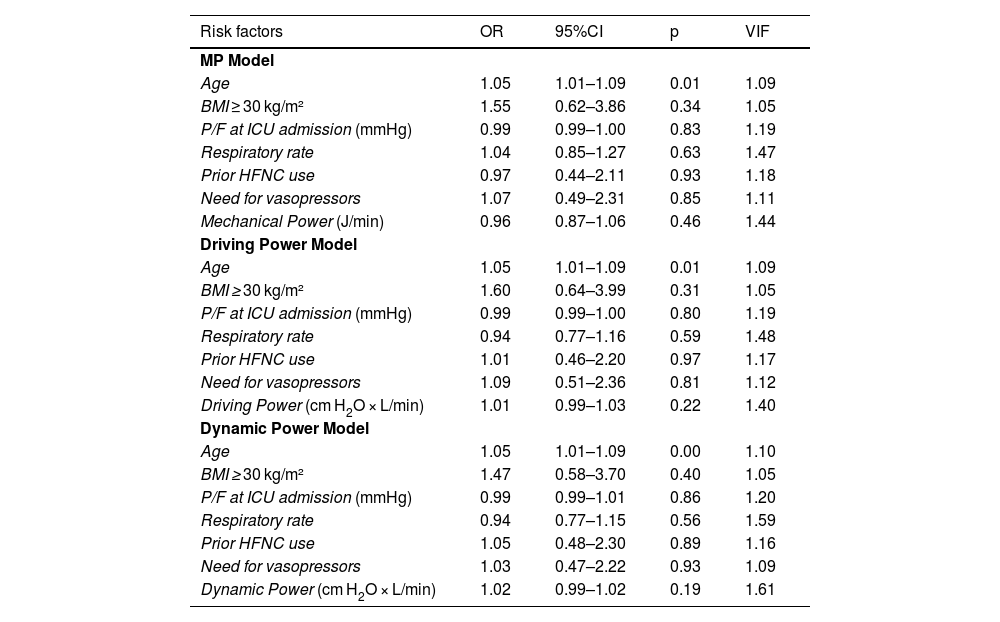
In the logistic regression analysis performed (Table 2), in the 3 adjusted models, only age proved to be a statistically significant independent predictor of mortality. Although body mass index (BMI) ≥ 30 kg/m2 showed a positive association with mortality, it did not reach statistical significance in any of the 3 adjusted models. Other factors such as the P/F ratio at ICU admission, respiratory rate, prior use of HFNC, and the need for vasopressors did not show a significant association with mortality in any of the models.
Risk factors associated with the 28-day mortality rate through multivariable logistic regression analysis.
| Risk factors | OR | 95%CI | p | VIF |
|---|---|---|---|---|
| MP Model | ||||
| Age | 1.05 | 1.01–1.09 | 0.01 | 1.09 |
| BMI ≥ 30 kg/m² | 1.55 | 0.62–3.86 | 0.34 | 1.05 |
| P/F at ICU admission (mmHg) | 0.99 | 0.99–1.00 | 0.83 | 1.19 |
| Respiratory rate | 1.04 | 0.85–1.27 | 0.63 | 1.47 |
| Prior HFNC use | 0.97 | 0.44–2.11 | 0.93 | 1.18 |
| Need for vasopressors | 1.07 | 0.49–2.31 | 0.85 | 1.11 |
| Mechanical Power (J/min) | 0.96 | 0.87–1.06 | 0.46 | 1.44 |
| Driving Power Model | ||||
| Age | 1.05 | 1.01–1.09 | 0.01 | 1.09 |
| BMI ≥ 30 kg/m² | 1.60 | 0.64–3.99 | 0.31 | 1.05 |
| P/F at ICU admission (mmHg) | 0.99 | 0.99–1.00 | 0.80 | 1.19 |
| Respiratory rate | 0.94 | 0.77–1.16 | 0.59 | 1.48 |
| Prior HFNC use | 1.01 | 0.46–2.20 | 0.97 | 1.17 |
| Need for vasopressors | 1.09 | 0.51–2.36 | 0.81 | 1.12 |
| Driving Power (cm H2O × L/min) | 1.01 | 0.99–1.03 | 0.22 | 1.40 |
| Dynamic Power Model | ||||
| Age | 1.05 | 1.01–1.09 | 0.00 | 1.10 |
| BMI ≥ 30 kg/m² | 1.47 | 0.58–3.70 | 0.40 | 1.05 |
| P/F at ICU admission (mmHg) | 0.99 | 0.99–1.01 | 0.86 | 1.20 |
| Respiratory rate | 0.94 | 0.77–1.15 | 0.56 | 1.59 |
| Prior HFNC use | 1.05 | 0.48–2.30 | 0.89 | 1.16 |
| Need for vasopressors | 1.03 | 0.47–2.22 | 0.93 | 1.09 |
| Dynamic Power (cm H2O × L/min) | 1.02 | 0.99–1.02 | 0.19 | 1.61 |
BMI, body mass index; P/F, ratio of arterial partial pressure of oxygen to the fraction of inspired oxygen.
Data expressed as odds ratios (OR) with their 95% confidence intervals (CI95%). The p-value was calculated using logistic regression analysis. The diagnosis of multicollinearity is shown with the Variance Inflation Factor (VIF).
Our analysis proves that the parameterization of mechanical ventilation in obese patients during the SARS-CoV-2 pandemic led to a higher Dynamic Power than in the rest of the patients, without this finding conditioning an increase in MP or an effect on 28-day mortality at the ICU setting.
In this context, former studies have demonstrated that the overall compliance of the respiratory system decreases in obese patients due to a decrease in chest wall compliance, while lung compliance remains unchanged.9 Obese patients may require higher PEEP values during mechanical ventilation to counteract the weight load imposed on it. This situation requires a higher energy load: Dynamic Power. Various published studies on PEEP values used in these patients describe the need for a mean PEEP between 11 and 18 cm H2O to achieve total recruitment of collapsed lung tissue.8
Our analysis was unable to detect a statistically significant association between energy load variables and 28-day mortality in this patient cohort. Despite the theoretical relevance of these variables and their potential impact on clinical outcomes, our study may lack sufficient statistical power to detect this effect on short-term mortality.
The confirmation of these findings would raise questions about the clinical utility of these energy load measures in predicting outcomes in critically ill obese patients. It is possible that other factors, such as the severity of the underlying disease, response to treatment, and comorbidities, have a more significant impact on mortality than energy load measures per se.
Consequently, these findings highlight the need for additional research to better understand the relationship between energy load in mechanical ventilation and clinical outcomes, as well as to identify more predictive biomarkers and clinical variables of mortality in critically ill patients.10
Our results in patients with acute respiratory distress syndrome (ARDS) raise questions about their extrapolation to the “typical” ARDS population, especially in those with bacterial pneumonia and intra-abdominal disease. Understanding the unique viral pathogenesis of SARS-CoV-2 underlies in the physiological differentiation between C-ARDS and non-COVID-19-related ARDS. Proinflammatory responses, closely associated with pulmonary vascular endothelial injury and immunothrombosis, show significant discrepancies between both types of ARDS.11
Authors’ contributionsAll the signatory authors met the authorship requirements and declared no conflicts of interest whatsoever.
Alejandro González-Castro: ideation, preparation, and drafting of the manuscript. Elena Cuenca Fito: data mining. Yhivian Peñasco: preparation, proofreading. Carmen Huertas: database cleaning. Aurio Fajardo: proofreading.