Medication errors, potentially causing harm and causing harm, increase significantly in newborns cared for in intensive care settings. In this sense, this work carries out a systematic review to analyze the most current evidence in relation to medication errors in neonatal intensive care, discussing the topics that refer to health technology from smart pumps, cost-effectiveness of medications, the practice of nursing professionals on the medication administration process and quality improvement models. In this way, it could be considered a useful tool to promote quality and safety in neonatal intensive care.
Los errores de medicación, con potencial de causar daño y con daño, aumentan significativamente en los recién nacidos atendidos en ambientes de cuidados intensivos. En este sentido, este trabajo lleva a cabo una revisión sistemática para analizar de la evidencia más actual con relación a los errores de medicación en los cuidados intensivos neonatales, discutiendo los temas que hacen referencia a la tecnología sanitaria a partir de las bombas inteligentes, el costo-efectividad de los medicamentos, la práctica de los profesionales de enfermería sobre el proceso de administración de medicamentos y los modelos de mejora de la calidad. De este modo, podría ser considerada una herramienta útil para promover la calidad y seguridad en los cuidados intensivos neonatales.
Medication errors (MEs) represent approximately one-third of all errors in medical care.1 In this regard, it is also estimated that one-third of all MEs in pediatrics and neonatal care occur in the course of medication preparation and administration, and that 18.7% to 56.0% of all adverse events among hospitalized patients are attributable to avoidable MEs.2
The administration of medications in clinical practice is a complex process with multiple possibilities of error.3 The intensive care setting is characterized by frequent handling of drugs, and thus stands out in this respect.
Medication errors are frequent in neonates,4–6 since these patients are vulnerable due to many factors such as physiological immaturity and rapid changes in body weight that affect the dosing of medications based on patient weight.7,8
Medication error can occur in any of the phases of drug use: selection and management, prescription, validation, preparation, dispensation, administration and follow-up. The administration of drugs is normally carried out by nurses based on a specific “5 correct” items protocol (correct patient, drug, dose, time and administration route).9 Many factors can have a negative impact on this process, such as simultaneous demands and interruptions, omissions in procedure, lack of clinical experience, and factors related to the system.10,11 Different studies have confirmed that errors recorded during drug administration can be avoided, thus highlighting the important role played by nursing professionals in the system for promoting patient safety.
The present systematic review was carried out to describe the most current evidence on MEs in neonatal intensive care.
MethodsSearch strategyThe present study followed the recommendations of the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA).12,13 The articles included in the study were identified by searching the following academic databases: Cumulative Index to Nursing and Allied Health Literature (CINAHL), SCOPUS, Scielo, Medline/PubMed, Cochrane, and Google Scholar. The literature search covered the period between 17 November to 23 December 2023. The following MeSH terms were used: “Medication Errors”, “Intensive Care, Neonatal”, combined with the Boolean operators AND, and obtaining the corresponding search chain (("Medication Errors"[MeSH]) AND "Intensive Care, Neonatal"[MeSH]).
The PICO question was applied, with the following elements:
- -
Participants/population: in order to be eligible for inclusion, the studies were required to specifically address the neonatal intensive care population.
- -
Interventions: the review focused on the analysis of MEs in neonatal intensive care.
- -
Comparators/controls: studies comparing MEs in neonates versus other ages (pediatric patients or adults) were considered.
- -
Outcomes: the specific outcomes of interest and data eligible for inclusion were: healthcare technology, models of quality improvement, cost-effectiveness and practice in the medication administration process.
The articles of interest were those describing the most current evidence on MEs in neonatal intensive care. We only considered full-text publications, in any language, and published in academic journals with peer review in the period between 2019 and 2023. With regard to the type of study design, we included randomized and controlled clinical trials, retrospective studies (case-control studies), cross-sectional, observational, retrospective cohort studies, cost studies, randomized prospective studies (cohorts), qualitative studies and quasi-experimental studies. This search window was used in adherence to the literature review recommendations.13 Duplicate articles, clinical cases and articles describing the analysis of MEs in areas other than neonatal intensive care were excluded.
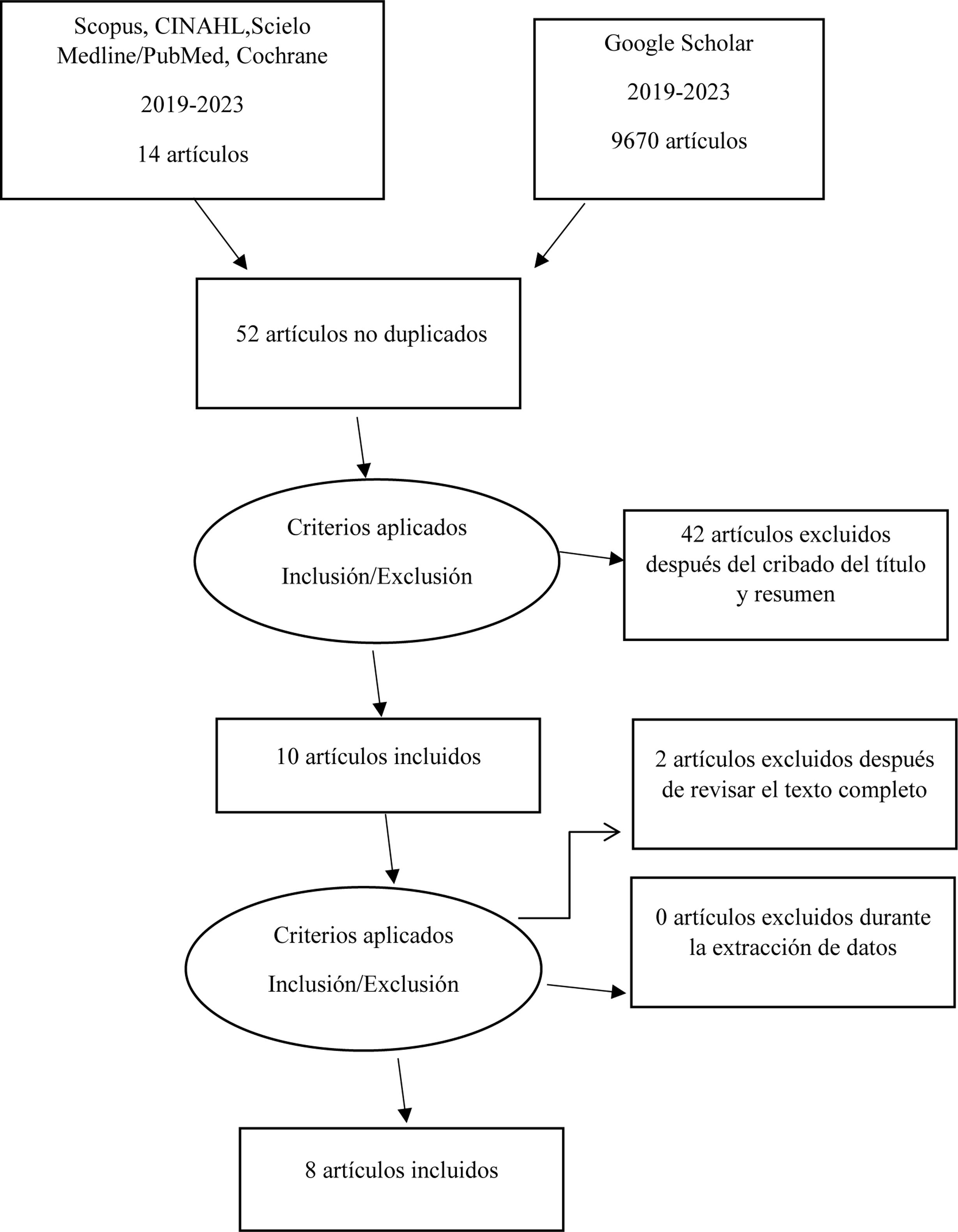
Data extraction and analysisTwo investigators initially analyzed the references separately, based on the title and abstract, and those that were seen to meet the inclusion criteria were subjected to independent full-text evaluation for possible inclusion. Agreement between the investigators was 90%, and those publications failing to reach such concordance were discarded. The selected articles were included on an independent basis by phases (summary, consensus and checking of results), with a recording of the year of publication, journal, country, sample size, description of the intervention, results and conclusions. The quality of the studies was assessed according to the risk of bias using the Cochrane tool.14 The following response variables in turn were extracted from the articles included in the systematic review: technological analysis, cost-effectiveness and practical analysis in relation to MEs in neonatal intensive care. Fig. 1 shows the reference search and extraction procedure (PRISMA flow chart).
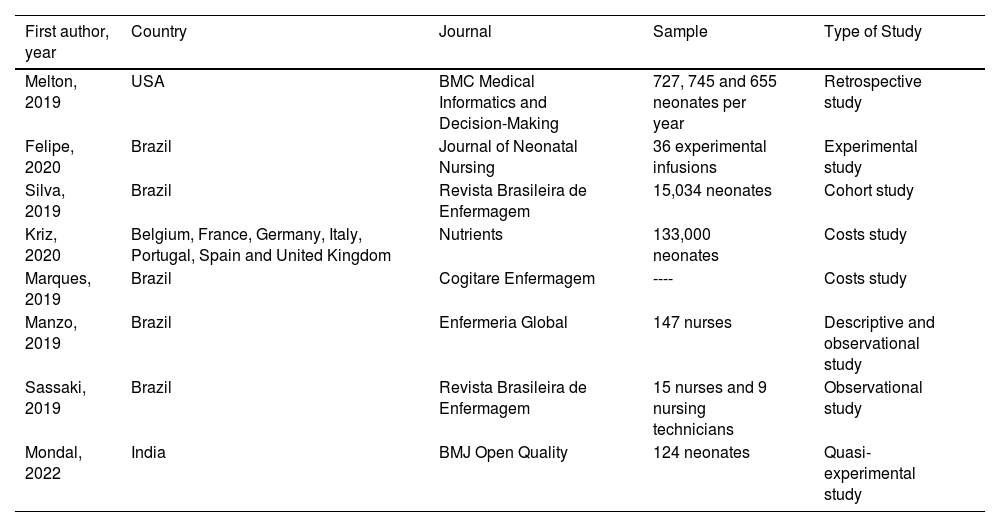
ResultsEight studies that met the inclusion criteria were carefully reviewed. Most of the studies were carried out in Brazil. The publication dates of the included articles were between 2019 and 2023. The study sample sizes in turn ranged between 24 and 133,000 participants. The characteristics of each included article are shown in Table 1.
Characteristics of the articles included in the study.
| First author, year | Country | Journal | Sample | Type of Study |
|---|---|---|---|---|
| Melton, 2019 | USA | BMC Medical Informatics and Decision-Making | 727, 745 and 655 neonates per year | Retrospective study |
| Felipe, 2020 | Brazil | Journal of Neonatal Nursing | 36 experimental infusions | Experimental study |
| Silva, 2019 | Brazil | Revista Brasileira de Enfermagem | 15,034 neonates | Cohort study |
| Kriz, 2020 | Belgium, France, Germany, Italy, Portugal, Spain and United Kingdom | Nutrients | 133,000 neonates | Costs study |
| Marques, 2019 | Brazil | Cogitare Enfermagem | ---- | Costs study |
| Manzo, 2019 | Brazil | Enfermeria Global | 147 nurses | Descriptive and observational study |
| Sassaki, 2019 | Brazil | Revista Brasileira de Enfermagem | 15 nurses and 9 nursing technicians | Observational study |
| Mondal, 2022 | India | BMJ Open Quality | 124 neonates | Quasi-experimental study |
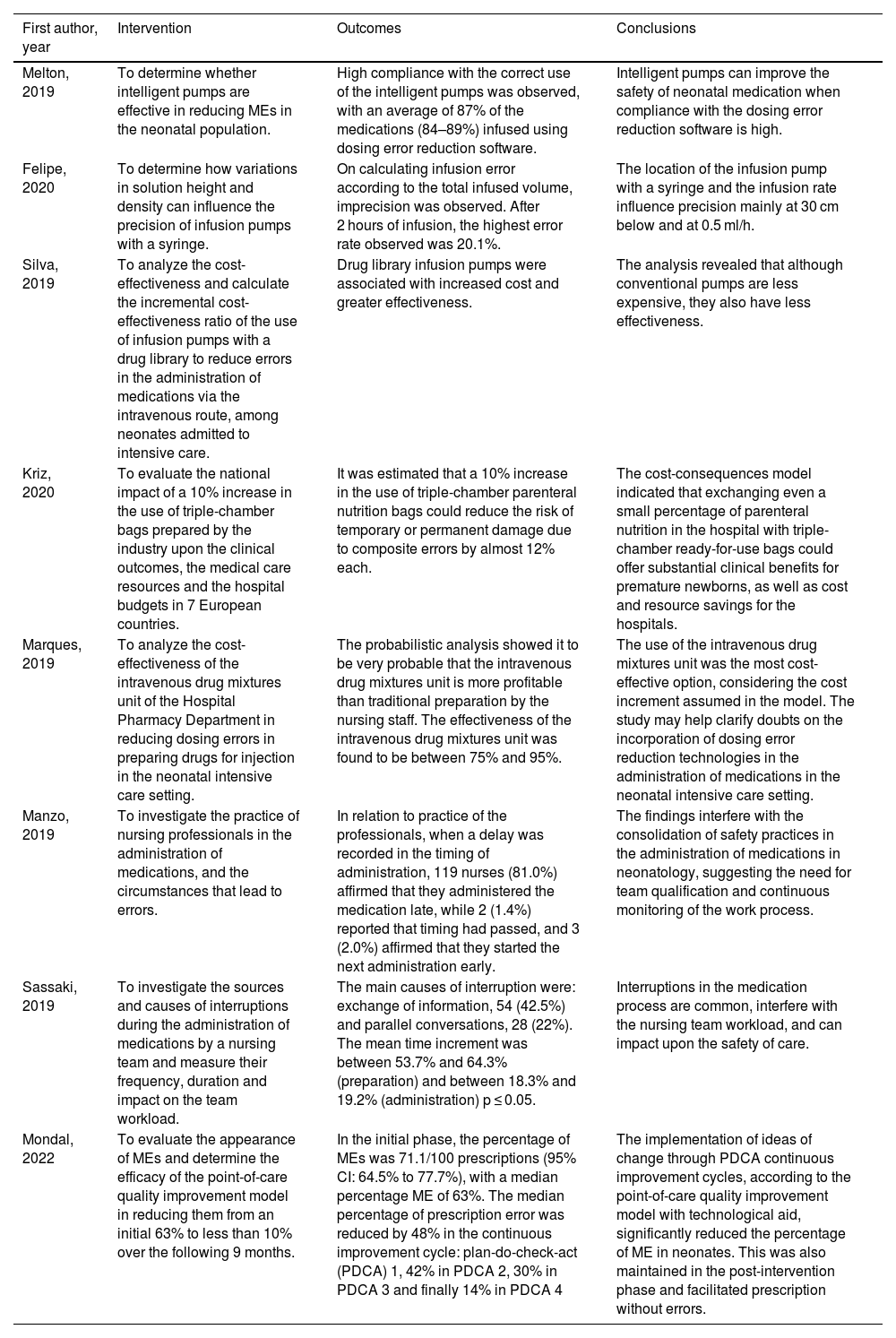
After analyzing the contents of the 8 included publications, observational, experimental and cost-effectiveness studies were seen to predominate. The main study tools comprised technological analysis, cost-effectiveness and practical analysis in relation to MEs in neonatal intensive care. Table 2 summarizes the results of each study.
Study topics and related evidence.
| First author, year | Intervention | Outcomes | Conclusions |
|---|---|---|---|
| Melton, 2019 | To determine whether intelligent pumps are effective in reducing MEs in the neonatal population. | High compliance with the correct use of the intelligent pumps was observed, with an average of 87% of the medications (84–89%) infused using dosing error reduction software. | Intelligent pumps can improve the safety of neonatal medication when compliance with the dosing error reduction software is high. |
| Felipe, 2020 | To determine how variations in solution height and density can influence the precision of infusion pumps with a syringe. | On calculating infusion error according to the total infused volume, imprecision was observed. After 2 hours of infusion, the highest error rate observed was 20.1%. | The location of the infusion pump with a syringe and the infusion rate influence precision mainly at 30 cm below and at 0.5 ml/h. |
| Silva, 2019 | To analyze the cost-effectiveness and calculate the incremental cost-effectiveness ratio of the use of infusion pumps with a drug library to reduce errors in the administration of medications via the intravenous route, among neonates admitted to intensive care. | Drug library infusion pumps were associated with increased cost and greater effectiveness. | The analysis revealed that although conventional pumps are less expensive, they also have less effectiveness. |
| Kriz, 2020 | To evaluate the national impact of a 10% increase in the use of triple-chamber bags prepared by the industry upon the clinical outcomes, the medical care resources and the hospital budgets in 7 European countries. | It was estimated that a 10% increase in the use of triple-chamber parenteral nutrition bags could reduce the risk of temporary or permanent damage due to composite errors by almost 12% each. | The cost-consequences model indicated that exchanging even a small percentage of parenteral nutrition in the hospital with triple-chamber ready-for-use bags could offer substantial clinical benefits for premature newborns, as well as cost and resource savings for the hospitals. |
| Marques, 2019 | To analyze the cost-effectiveness of the intravenous drug mixtures unit of the Hospital Pharmacy Department in reducing dosing errors in preparing drugs for injection in the neonatal intensive care setting. | The probabilistic analysis showed it to be very probable that the intravenous drug mixtures unit is more profitable than traditional preparation by the nursing staff. The effectiveness of the intravenous drug mixtures unit was found to be between 75% and 95%. | The use of the intravenous drug mixtures unit was the most cost-effective option, considering the cost increment assumed in the model. The study may help clarify doubts on the incorporation of dosing error reduction technologies in the administration of medications in the neonatal intensive care setting. |
| Manzo, 2019 | To investigate the practice of nursing professionals in the administration of medications, and the circumstances that lead to errors. | In relation to practice of the professionals, when a delay was recorded in the timing of administration, 119 nurses (81.0%) affirmed that they administered the medication late, while 2 (1.4%) reported that timing had passed, and 3 (2.0%) affirmed that they started the next administration early. | The findings interfere with the consolidation of safety practices in the administration of medications in neonatology, suggesting the need for team qualification and continuous monitoring of the work process. |
| Sassaki, 2019 | To investigate the sources and causes of interruptions during the administration of medications by a nursing team and measure their frequency, duration and impact on the team workload. | The main causes of interruption were: exchange of information, 54 (42.5%) and parallel conversations, 28 (22%). The mean time increment was between 53.7% and 64.3% (preparation) and between 18.3% and 19.2% (administration) p ≤ 0.05. | Interruptions in the medication process are common, interfere with the nursing team workload, and can impact upon the safety of care. |
| Mondal, 2022 | To evaluate the appearance of MEs and determine the efficacy of the point-of-care quality improvement model in reducing them from an initial 63% to less than 10% over the following 9 months. | In the initial phase, the percentage of MEs was 71.1/100 prescriptions (95% CI: 64.5% to 77.7%), with a median percentage ME of 63%. The median percentage of prescription error was reduced by 48% in the continuous improvement cycle: plan-do-check-act (PDCA) 1, 42% in PDCA 2, 30% in PDCA 3 and finally 14% in PDCA 4 | The implementation of ideas of change through PDCA continuous improvement cycles, according to the point-of-care quality improvement model with technological aid, significantly reduced the percentage of ME in neonates. This was also maintained in the post-intervention phase and facilitated prescription without errors. |
ME: medication error.
Melton et al.15 examined whether intelligent pumps are effective in reducing MEs in the neonatal population. These pumps prevented 160 attempts to exceed the strict maximum dose (between 7 and 29 times the maximum dose), and resulted in the reprogramming or cancellation of 2093 infusions.
Felipe et al.16 found that variations in the height of location and density of the solutions can influence the precision of syringe infusion pumps. After two hours of infusion, loss of precision was observed at infusion rates of under 0.5 ml/h, with a significant influence of the height at which the infusion pump was placed (p < 0.001). At an infusion rate of 10.0 ml/h, there were differences between saline solution versus parenteral nutrition positioned at the same height (p < 0.004) and 30 cm above (p < 0.001).
Silva et al.17 analyzed the cost-effectiveness and calculated the incremental cost-effectiveness ratio of the use of infusion pumps with drugs to reduce errors in the administration of intravenous medications in neonatal intensive care. The decision tree showed that infusion pumps with the drug guide can be the best strategy for avoiding errors in the intravenous administration of medications, despite the increased cost involved.
Kriz et al.18 in turn evaluated the national impact of a 10% increase in the use of triple-chamber bags prepared by the industry upon the clinical outcomes, medical care resources and hospital budgets in 7 European countries. The reference composition methods were estimated to be 43% manual pharmacy, 16% automated pharmacy, 22% in the ward, 9% contracted to third parties, 3% supplied by the industry in non-three-chamber bags, and 7% in three-chamber bags. An increased use of triple-chamber bags would change these figures to 39%, 15%, 18%, 9%, 3% and 17%, respectively.
Marques et al.19 analyzed the cost-effectiveness of the intravenous drug mixtures unit of the Hospital Pharmacy Department in reducing dosage errors in preparing medications for injection in neonatal intensive care. The model predicted that the intravenous drug mixtures unit was cost-effective, showing a mean effectiveness of 0.96 in avoiding dosage errors in the administration of intravenous drugs, with an incremental cost-effectiveness ratio of R$ 26,785.61.
Manzo et al.20 investigated nursing professional practice in the administration of medications, and the circumstances leading to error. The identified weaknesses were referred to the double checking of medications, the administration of drugs prepared by a colleague, delays and a lack of verification of the prescriptions. The most common errors corresponded to wrong dosages, and environmental factors were identified as the critical point.
Sassaki et al.21 in turn investigated the sources and causes of interruptions during the drug administration process on the part of the nursing team, and measured their frequency, duration and impact on the team workload. In each round, the number of interruptions ranged from 1–7, totaling 127. The interruptions mainly occurred during the preparation phase (n = 97, 76.4%). In turn, the main sources of interruption were the nursing staff (n = 48, 37.8%) and self-interruptions (n = 29, 22.8%).
Lastly, Mondal et al.22 evaluated the prevalence of MEs and determined the efficacy of the point-of-care quality improvement model in reducing such errors from an initial incidence of 63% to less than 10% over the 9 months following its implementation. The study showed the total errors to be reduced to 10.4/100 prescriptions (p < 0.005), with significant decreases in errors referred to the dosage, timing, interval, preparation and infusion rate of medications in the prescriptions of the post-intervention phase.
DiscussionIt is important to consider and analyze MEs in neonatal intensive care. Three studies15–17 analyzed healthcare technologies involving intelligent infusion pumps and their effectiveness. Two studies18,19 performed cost-effectiveness analyses of medications in the reduction of dosing errors. Another two articles articles20,21 examined healthcare professional practice in the administration of medications. One study22 analyzed the appearance of ME and determined the efficacy of a quality improvement model.
Melton et al.15 found that the nursing staff correctly used intelligent pumps and programmed most infusions using the medications guide with a dosage error reduction software application. As in other studies, such a high compliance rate was found to be crucial for the effective use of intelligent pumps.23
Felipe et al.16 reported an association between the performance of the syringe infusion pump, the type of solution infused, and the height at which the device was positioned. The most important risk factors associated with the clinical use of syringe infusion pumps include overdose or an insufficient dose as a result of imprecision in the amount of medication infused, leading to adverse events such as oversedation, respiratory depression, or significant blood pressure fluctuations.24 The incidence rate of errors of this kind is particularly important in the neonatal population since these patients require more precise doses and low infusion volumes.25 However, as seen in this study, syringe infusion pumps lost precision when a low infusion rate was established, and an extreme 20.1% incidence of errors was recorded in evaluating the total infused volume.
Silva et al.17 found that infusion pumps with a drug library imply greater cost but also afford greater effectiveness. Some studies have obtained inconclusive results regarding the effectiveness of intelligent pumps in reducing adverse drug reactions, reporting that there is no reduction of the risk associated with the implantation of intelligent pumps,26 though other studies have concluded that this technology can avoid dosing errors and thus increase patient safety.27
The cost-effectiveness analysis published by Kriz et al.18 showed that even a modest 10% increase in the use of ready-to-use three-chamber bags in premature infants receiving parenteral nutrition could substantially improve the clinical outcomes, reduce work time, and lessen the impact on hospital budgets. It is important to note that the incidences of significant and severe damage due to composite errors could decrease markedly between 10–13%, and the sepsis rate between 2–3%. As in other studies, the risk of infection can be reduced directly by limiting the number of manipulation steps involved.28
The results of Marques et al.19 should alert nursing professionals and healthcare management bodies to reconsider the use of traditional methods for the preparation of intravenous medications since they question the preparation of drugs for injection by nursing teams. In this sense, the American Society of Health-System Pharmacists points to the distribution of single-dose formulations and the use of the pharmacy intravenous drug preparation system as important recommendations for preventing MEs in a hospital. Studies conducted by the Instituto da Criança showed that with the availability of single-dose formulations, pharmacies can reduce internal consumption by up to 35%.29 In the case of premature infants, implementation of the intravenous drugs mixture unit affords greater treatment safety and quality.30
Manzo et al.20 underscored that the unique characteristics and complexity of therapy in neonatology make it extremely important to continuously increase scientific knowledge and capacitation in this field.31 However, this study found that 63.9% of the participants reported not having participated in courses or conferences on the preparation and administration of drugs in the last year, and that most had doubts regarding the action of medications. Thus, in order to ensure safety and quality, it is necessary to revise the working processes based on the scientific evidence, and to make sure that the professionals (both leaders and those under their supervision) are capacitated and duly qualified.
Sassaki et al.21 highlighted the preparation phase as being critical for interruptions among the nursing staff (48%) and nursing technicians (28.3%), in concordance with the observations of another study (72.7%).32 It must be mentioned that when the professional is interrupted, his or her attention focuses on other demands,33 and such distractions account for almost 50% of all MEs.34
In the study published by Mondal, et al.,22 a considerable number of errors were related to incorrect timings or intervals. A South African study also found the most common problem to be an incorrect drug prescription interval, associated mainly with antibiotic prescription, since the interval of certain antibiotics varies according to the age of the newborn.35 Although the different error indicators in this study were not significantly correlated to the gestational age of the neonates, other studies have pointed to prematurity as a significant determinant in the appearance of MEs, possibly due to longer hospital stays or the use of more medications or complex calculations as causal factors.36
Observational,20,21 experimental16,22 and cost analysis18,19 studies were the most widely used designs. Among the different articles, healthcare technology analyses and cost-effectiveness and practice studies referred to MEs in neonatal intensive care were the main topics. At a practical level, mention should be made of the observation that decision-making regarding the incorporation of technologies could reduce dosage errors in the administration of medications in neonatal intensive care.
The 8 reviewed studies involved different interventions and different outcome measures – a fact that complicates the drawing of conclusions as to which intervention is most effective. Future research on this subject should take into account the aspects highlighted by the World Health Organization (WHO) to secure safer patient care, seeking causes, proposing solutions, and evaluating impacts. We thus advise the conduction of multicenter studies using the same measurement tools to determine the effectiveness of interventions in terms of specific outcomes.
The present review has several limitations. Because of the observed methodological heterogeneity, the characteristics of the participants and the representativeness of the studies, it is difficult to draw conclusions and generalize the findings to other contexts. Lastly, although extensive database and manual searches were made, some relevant studies inadvertently might have been excluded.
ConclusionsThe present systematic review provides information on the current evidence on the analysis of MEs in neonatal intensive care. The implications of the findings for patient safety refer to the need to apply healthcare technology (intelligent pumps) and new models of quality improvement, as well as improvements in the administration of medications on the part of the nursing staff. In this sense, the study could be a useful tool for promoting treatment safety and quality in neonatal intensive care.
In addition, further studies are needed, with the adoption of strategies by the healthcare administrations/organizations in neonatal intensive care, including the analysis of their applicability in different contexts.
Financial supportNone.
Author contributionsF.M.E.R: Conceptualization, supervision, review and editing of the text. L.P.F: Data search, draft preparation and synthesis. All signing authors reviewed the manuscript, made relevant contributions, and agreed to the final version.
Conflicts of interestNone.