High concentrations of caspase-8 (main initiator caspase of apoptosis extrinsic pathway) have been found in brain tissue from traumatic brain injury patients and in blood of patients with different diseases. However, there are not data on blood caspase-8 concentrations in ischemic stroke patients. Therefore, the objective of this study was to determine whether there is an association between blood caspase-8 concentrations and the probability and speed of mortality at 30 days in patients with malignant middle cerebral artery infarction (MMCAI).
DesignObservational prospective study.
SettingFive Intensive Care Units (ICU).
PatientsPatients with severe malignant middle cerebral artery infarction (MMCAI) defined as acute infarction in more than of 50% of that territory and Glasgow Coma Scale (GCS)<9.
InterventionsDetermination of serum caspase-8 levels when MMCAI was diagnosed.
Main variables of interestMortality at 30 days and time until this event.
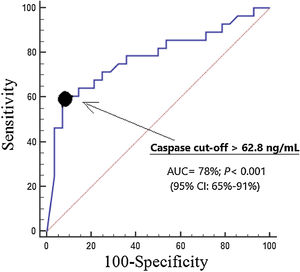
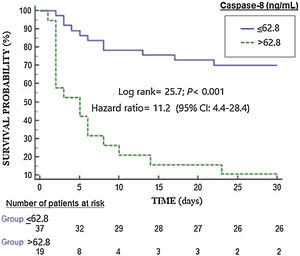
ResultsSevere MMCAI patients (n=28) compared to survivor patients (n=28) showed higher serum caspase-8 concentrations (p<0.001), lower platelet count (p=0.01) and lower GCS (p=0.002). We found an area under the curve for mortality prediction of 78% (95% CI=65%–91%; p<0.001) by serum caspase-8 levels. Kaplan–Meier analysis found higher mortality rate in patients with serum caspase-8 levels >62.8ng/mL (hazard ratio=11.2; 95% CI=4.4–28.4; p<0.001).
ConclusionsThe association of high blood caspase-8 concentrations with the rate and the velocity of 30-day mortality in MMCAI patients is the main new finding of our study.
Se han encontrado altas concentraciones de caspasa-8 (principal caspasa iniciadora de la vía extrínseca de apoptosis) en el tejido cerebral de pacientes con traumatismo craneoencefálico y en la sangre de pacientes con diferentes enfermedades. Sin embargo, no hay datos sobre las concentraciones sanguíneas de caspasa-8 en pacientes con ictus isquémico. Por tanto, el objetivo de este estudio fue determinar si existe una asociación entre las concentraciones sanguíneas de caspasa-8 y la probabilidad y velocidad de mortalidad a 30días en pacientes con infarto maligno de la arteria cerebral media (MMCAI).
DiseñoObservacional y prospectivo.
ÁmbitoCinco unidades de cuidados intensivos (UCI).
PacientesPacientes con MMCAI grave definido como infarto agudo en más del 50% de ese territorio y escala de coma de Glasgow (GCS)<9.
IntervencionesDeterminación de niveles séricos de caspasa-8 cuando se diagnosticó el MMCAI grave.
Variables de interés principalMortalidad hasta los 30dias y tiempo hasta este evento.
ResultadosLos pacientes fallecidos (n=28) en comparación con los supervivientes (n=28) mostraron mayores concentraciones séricas de caspasa-8 (p<0,001), menor recuento plaquetario (p=0,01) y menor GCS (p=0,002). Encontramos un área bajo la curva para la predicción de mortalidad del 78% (IC 95%: 65-91%; p<0,001) por los niveles séricos de caspasa-8. El análisis de Kaplan-Meier encontró una mayor tasa de mortalidad en pacientes con niveles séricos de caspasa-8>62,8ng/mL (hazard ratio: 11,2; IC 95%: 4,4-28,4; p<0,001).
ConclusionesLa asociación de elevadas concentraciones sanguíneas de caspasa-8 con la tasa y velocidad de mortalidad a 30días en pacientes con MMCAI es el principal hallazgo nuevo de nuestro estudio.
Many deaths, disabilities and health resources consumption are produced by ischemic stroke.1 In addition to the primary cerebral damage due to vasculature obstruction, a secondary cerebral damage due to apoptosis could appear in ischemic stroke.2–6 There have been apoptotic changes in brain tissue samples from ischemic stroke patients.7–12
The activation of apoptosis extrinsic pathway occurs when a ligand of the tumor necrosis factor ligand superfamily (TNFSF) binds with its receptor of tumor necrosis factor membrane receptors superfamily (TNFRSF) generating a death signal. Then, this death signal activates caspase-8 (which is an initiator caspase) and caspase-8 activated is responsible to activate caspase-3, which produce apoptotic cellular damage due to that is a main executor caspase.2–6
In one study were found higher caspase-8 concentrations from brain tissue samples removed from traumatic brain injury patients for surgical management of severe intracranial hypertension compared with postmortem brain tissue samples from control subjects.13 Increased blood caspase-8 concentrations have been found in patients with systemic lupus erythematosus,14,15 in patients with metabolic syndrome and non-alcoholic fatty liver disease,16 and in septic patients with worse prognosis.17 However, there are not data on blood caspase-8 concentrations in ischemic stroke patients. We hypothesize that high blood caspase-8 concentrations increase the probability and speed of mortality. Therefore, the objective of this study was to determine whether there is an association between blood caspase-8 concentrations and the probability and speed of mortality at 30 days in patients with malignant middle cerebral artery infarction (MMCAI).
MethodsDesign and subjectsThe study was performed with the approval by the research ethic committee of all hospitals, and with the written and signed informed consent by some relative of each patient. This observational and prospective study was carried out during 2016 and 2017 in the Intensive Care Units of 5 hospitals located in Spain: H. Insular de Las Palmas de Gran Canaria, H. General de La Palma, H. Universitario de Canarias, H. Universitario Dr. Negrín de Las Palmas de Gran Canaria and H. Universitario Nuestra Señora de Candelaria.
We included patients with severe MMCAI. Glasgow Coma Scale (GCS)18 <9 points was the criterion to define severe, and findings of an acute infarction at least in 50% of that territory in computed tomography was the criterion to define malignant. We excluded patients with limited interventions order at hospital admission, inflammatory disease, pregnancy, age less than 18 years or malignant disease.
The following variables were recorded at the moment of MMCAI diagnosis: age, sex, arterial hypertension, diabetes mellitus, GCS, temperature, fraction inspired of oxygen (FiO2), pressure of arterial oxygen (PaO2), sodium, lactic acid, glycemia, creatinine, platelets, hemoglobin, fibrinogen, international normalized ratio (INR), activated partial thromboplastin time (aPTT), leukocytes, thrombolysis, volume infarction, midline shift and hemorrhagic transformation. Acute Physiology and Chronic Health Evaluation II (APACHE II) score19 was calculated with worst recorded values during the first 24h after the inclusion on the study. We calculated the infarct volume from computed tomography by the formula 0.5×A×B×C (where A and B are the largest perpendicular diameters and C is the cranio-caudal diameter estimated by the product of the number of cuts in which the hypodensity appears multiplied by their thickness).20 We also registered decompressive craniectomy. Main variables of interest were mortality at 30 days and time until this event.
Blood sample collection and serum caspase-8 analysisAt moment of severe MMCAI diagnosis were collected blood samples, and until the determination moment were frozen at −80°C. Serum caspase-8 concentrations were measured with the Human Caspase 8 ELISA Kit (Elabscience, Houston, Texas, United States), which has a detection limit of 0.10ng/mL, a variation coefficient intra-assay <6%, and a variation coefficient inter-assay <8%.
Statistical methodsWe report continuous variables with medians with interquartile ranges, and categorical with frequencies with percentages. We compare continuous variables between survivor and non-survivor patients with Wilcoxon–Mann–Whitney test, and categorical variables with chi-square test. A multiple logistic regression analysis, including the variables with the lowest p-value in the comparison between non-surviving and surviving patients and other key clinical variables, was performed to test the association of those variables with mortality; and odds ratio was provided for each variable. A receiver operating characteristic (ROC) curve analysis was carried out to test the mortality prognostic capability of serum caspase-8 concentrations. We performed Kaplan–Meier analysis including patients with higher and lower serum caspase-8 levels than 62.8ng/mL (cut-off selected by Youden J index) and both curves were compared with log-rank test. We use a Cox simple regression analysis to estimate the hazard ratio of 30-day mortality in patients with higher and lower serum caspase-8 levels than 62.8ng/mL, the event was mortality and the dependent variable was the time to event (mortality). We tested proportional risks assumption in Cox simple regression analysis. Statistical analyses were performed by means of SPSS 17.0 (SPSS Inc., Chicago, IL, USA) and MedCalc Statistical Software version 19.4.0 (MedCalc Software Ltd, Ostend, Belgium).
ResultsNon-survivor patients (n=28) compared to survivor patients (n=28) showed higher serum caspase-8 concentrations (p<0.001), lower platelet count (p=0.01) and lower GCS (p=0.002) (Table 1).
Clinical and biochemical characteristics of survivor and non-survivor patients.
| Survivors (n=28) | Non-survivors (n=28) | p-value | |
|---|---|---|---|
| Gender female – number (%) | 13 (46.4) | 11 (39.3) | 0.79 |
| Arterial hypertension – number (%) | 17 (60.7) | 16 (57.1) | 0.99 |
| COPD – number (%) | 1 (3.6) | 1 (3.6) | 0.99 |
| Chronic renal failure – number (%) | 2 (7.1) | 2 (7.1) | 0.99 |
| Heart failure – number (%) | 1 (3.6) | 1 (3.6) | 0.99 |
| Diabetes mellitus – number (%) | 3 (10.7) | 7 (25.0) | 0.30 |
| Age (years) – median (p 25–75) | 60 (47–68) | 61 (52–70) | 0.42 |
| Temperature (°C) – median (p 25–75) | 36.4 (35.7–37.0) | 37.0 (36.0–37.6) | 0.08 |
| GCS score – median (p 25–75) | 8 (7–8) | 6 (3–7) | 0.002 |
| APACHE-II score – median (p 25–75) | 20 (16–26) | 22 (20–28) | 0.14 |
| Glycemia (g/dL) – median (p 25–75) | 128 (98–165) | 135 (89–161) | 0.99 |
| Creatinine (mg/dL) – median (p 25–75) | 0.80 (0.60–1.18) | 1.05 (0.78–1.33) | 0.08 |
| Fibrinogen (mg/dL) – median (p 25–75) | 449 (419–541) | 495 (378–641) | 0.99 |
| Sodium (mEq/L) – median (p 25–75) | 140 (136–145) | 140 (138–143) | 0.99 |
| Lactic acid (mmol/L) – median (p 25–75) | 1.30 (1.00–1.70) | 1.60 (1.00–2.95) | 0.15 |
| Caspase-8 (ng/mL) – median (p 25–75) | 17.3 (8.0–42.4) | 73.0 (28.2–99.9) | <0.001 |
| Hemoglobin (g/dL) – median (p 25–75) | 12.0 (11.2–14.8) | 12.9 (10.8–14.8) | 0.93 |
| Leukocytes*103/mm3 – median (p 25–75) | 12.8 (9.8–17.4) | 13.8 (9.1–17.7) | 0.86 |
| Platelets*103/mm3 – median (p 25–75) | 210 (166–282) | 172 (129–207) | 0.01 |
| INR – median (p 25–75) | 1.07 (1.01–1.21) | 1.20 (1.05–1.31) | 0.08 |
| aPTT (seconds) – median (p 25–75) | 28 (26–30) | 27 (26–33) | 0.91 |
| PaO2 (mmHg) – median (p 25–75) | 137 (102–221) | 113 (85–143) | 0.22 |
| PaO2/FiO2 ratio – median (p 25–75) | 284 (205–375) | 240 (173–304) | 0.07 |
| Left hemisphere of cerebral infarction – number (%) | 12 (42.9) | 13 (46.4) | 0.99 |
| Volume infarction (cc) – median (p 25–75) | 173 (90–234) | 208 (115–288) | 0.40 |
| Midline shift (mm) – median (p 25–75) | 6 (2–11) | 8(2–15) | 0.52 |
| Hemorrhagic transformation – number (%) | 5 (17.9) | 5 (17.9) | 0.99 |
| Thrombolysis – number (%) | 10 (35.7) | 7 (25.0) | 0.56 |
| Decompressive craniectomy – number (%) | 7 (25.0) | 5 (17.9) | 0.75 |
COPD=Chronic Obstructive Pulmonary Disease; GCS=Glasgow Coma Scale; APACHE II=Acute Physiology and Chronic Health Evaluation; INR=international normalized ratio; aPTT=activated partial thromboplastin time; PaO2=pressure of arterial oxygen; FiO2=fraction of inspired oxygen.
The survival time of patients that dead during the first 30 day was 5 (2–8) days, and 16 from 28 patients (57.1%) dead in the first 5 days of MMCAI diagnosis. One patient that dead due to refractory septic shock secondary to nosocomial pneumonia survived 17 days. The 11 patients that dead due to sudden cardiac death survived 6 (5–8) days. The 16 patients that dead due to brain death secondary to refractory intracranial hypertension survived 3 (2–5) days. Limitation of vital support during ICU stay was not stablished in any patient. Any patient showed nosocomial infection at moment of inclusion in the study.
We found an area under the curve for mortality prediction of 78% (95% CI=65%–91%; p<0.001) by serum caspase-8 levels (Fig. 1). The cut-off of serum caspase-8 levels >62.8ng/mL had 61% of sensitivity (41%–79%), 93% of specificity (77%–99%), 8.5 of positive likelihood ratio (2.2–33.4), 0.4 of negative likelihood ratio (0.3–0.7), 90% of positive predictive value (68%–97%) and 70% of negative predictive value (60%–79%) for mortality prediction.
Kaplan–Meier analysis found higher mortality rate in patients with serum caspase-8 levels >62.8ng/mL (hazard ratio=11.2; 95% CI=4.4–28.4; p<0.001) (Fig. 2). We found that 11 of 37 patients (29.7%) showing serum caspase-8 levels ≤62.8ng/mL death during the first 30 days, and that 17 of 19 patients (89.5%) showing serum caspase-8 levels>62.8ng/mL death during the first 30 days (p<0.001).
We found in the multiple regression analysis an association between serum caspase-8 levels and mortality (OR=1.056; 95% CI=1.024–1.090; p=0.001) controlling for GCS and for platelet count (Table 2).
Multiple logistic regression analysis to predict 30-day mortality.
| Variable | Odds ratio | 95% confidence interval | p |
|---|---|---|---|
| First regression model | |||
| Glasgow Coma Scale (points) | 0.502 | 0.319–0.789 | 0.003 |
| Platelet count (each 1000/mm3) | 0.987 | 0.975–0.998 | 0.02 |
| Serum caspase-8 (ng/mL) | 1.056 | 1.024–1.090 | 0.001 |
| Second regression model | |||
| Serum caspase-8 (ng/mL) | 1.054 | 1.007–1.104 | 0.03 |
| Glasgow Coma Scale (points) | 0.458 | 0.217–0.963 | 0.04 |
| Volume infarction (cc) | 1.000 | 0.988–1.013 | 0.95 |
| Third regression model | |||
| Serum caspase-8 (ng/mL) | 1.043 | 1.020–1.066 | <0.001 |
| APACHE-II (points) | 1.131 | 1.006–1.272 | 0.04 |
| Decompressive craniectomy (yes vs no) | 1.067 | 0.207–5.505 | 0.94 |
We have not found an association of serum caspase-8 levels with GCS (rho=−0.04, p=0.76), platelet count (rho=−0.15, p=0.28) and volume infarction (rho=0.19, p=0.39).
DiscussionWe believed that this is the first series reporting data about blood caspase-8 concentrations in ischemic stroke patients. The association of high blood caspase-8 concentrations with the rate and the velocity of 30-day mortality in MMCAI patients is the main new finding of our study.
According with Kleinbaum,21 we provided Odds ratio (OR) and hazard ratio (HR) due to that provides different visions of the relationship between predictor variables and the dependent variable. In logistic regression analysis the dependent variable is the event, meanwhile in Cox regression analysis the dependent variable is time to the event. The OR estimated by logistic regression (shown in Table 2) provides a static vision of the relationship between caspase-8 and mortality at a fixed time, regardless of what happened in the time course up to the event. The HR estimated by Cox regression (shown in Fig. 2) provides a dynamic vision, where the time until the event (death) occurs is taken into account as a dependent variable. Different studies may have the same OR but very different HR, and for this that we prefer to include it among the analyses.
We want to clarify that in our study the censored cases are the patients who reached the maximum follow-up time (30 days) without presenting the event (death) and that any patient was censored before 30 days, as is showed in Fig. 2.
As we recorded 28 non-surviving patients during the first 30 days in our study, we constructed three logistic regression models with only three predictor variables in each model to avoid over fitting effect. The variables included in the first model were GCS, platelet count and serum caspase-8 concentration due to those variables showed the lowest p-value in the comparison between non-surviving and surviving patients. The second and third models included serum caspase-8 concentration and other key clinical variables as APACHE-II, volume infarction and decompressive craniectomy. Possibly more variables could be included in one only model in a larger series. It is possible that we have not found an association between other variables (as volume infarction and other variables) and mortality due to the low sample size of our study.
Brain tissue samples obtained from patients underwent decompressive craniectomy due to management of life-threatening intracranial hypertension after traumatic brain injury compared to those obtained from patients who did not die due to brain trauma have showed higher caspase-8 expression predominately in neurons.13,22 In addition, in brain tissue samples obtained from patients who die due to cerebral infarction have been found high expression of caspase-3 (main executor caspase) in neurons.9–12 Besides, higher expression of caspase-9 (the initiator caspase of intrinsic apoptotic pathway) has been found in patients dead due to cerebral infarction that in subject controls.7,8 However, there not data about caspase-8 expression in brain tissue samples obtained from patients with cerebral infarction. We think, that according with the findings of those studies, neuron apoptosis could be the origin of caspase-8 in patients with cerebral infarction. We decided to determine serum caspase-8 levels due to that we had previously found an association between blood caspase-8 concentrations and mortality in septic patients.17
Caspase-8 is the main initiator caspase in the apoptosis extrinsic pathway and is activated due to the action of a death signal that is generated when some TNFRSF binds with its TNFSF ligand. Caspase-8 activates to caspase-3, the main executor caspase, producing the cellular apoptotic changes.2–6 Therefore, we think that those higher blood caspase-8 concentrations that we found in non-survivor patients could be representative of higher apoptosis extrinsic pathway activation in brain, leading to higher executor caspases activation, and leading finally to higher brain apoptotic degree and the death of patient.
Limitations in our study were the lack of serum caspase-8 levels determination in healthy subjects, of serum caspase-8 levels determination during the evolution of patients, of caspase-8 concentrations and apoptosis degree assessment in brain samples, and the lack to register other possible cerebral territories affected. Blood samples were taken during the first 4h of severe MMCAI diagnosis; however, the exact time when blood sample were taken was not registered. Thus, we do not know the exact time from clinical onset of cerebral infarction to the time of taking blood samples and the exact time from GCS<9 to the time of taking blood samples; and those facts could have influenced the serum caspase-8 levels. Our main variables of interest were mortality at 30 days and time until this event due to that 30-day mortality is a main variable of interest very used in critically ill patients; however, other main variables of interest could be used.
We think that nosocomial infection has not contributed on blood caspase-8 levels due to any patient showed nosocomial infection at moment of inclusion in the study. The number of patients that were excluded and that refused to participate in the study were not registered. Besides, patients with limited interventions order at hospital admission were excluded, and all those facts could have contributed on the conclusions of the study.
The administration of different agents has attenuated brain caspase-8 expression, cerebral apoptosis, brain infarction volume and neurological deficit in rat models of brain ischemia.23–29
Previously, we found higher oxidative damage of deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) in non-surviving than in surviving patients with MMCAI in a previous series of patients.30 Thus, non-survivor patients have higher DNA and RNA damage by oxidation and by apoptosis, and both mechanisms have been modulated in animal models of brain ischemia.
We believed, that according to the findings of our series with ischemic stroke patients and the findings with the inhibition of caspase-8 in brain ischemia animal models, could be interesting to research on the mortality prognosis capability of serum caspase-8 levels in ischemic stroke patients and to research on the use of caspase-8 inhibitors to improve the prognosis of ischemic stroke patients.
ConclusionsThe association of high blood caspase-8 concentrations with the rate and the velocity of 30-day mortality in MMCAI patients is the main new finding of our study.
FundingThis study was supported by a grant from Instituto de Salud Carlos III (PI-18-00500) (Madrid, Spain) and co-financed with Fondo Europeo de Desarrollo Regional (FEDER).
Author contributions- -
LL conceived, designed and coordinated the study, participated in acquisition and interpretation of data, and drafted the manuscript.
- -
MMM, LRG, JSV and JJC participated in acquisition of data.
- -
APC, AFGR and JJVJ carried out the determinations of serum caspase-8 levels.
- -
AJ participated in the interpretation of data.
All authors revised the manuscript critically for important intellectual content and made the final approval of the version to be published.
Conflicts of interestThe authors declare that they have no conflicts of interest.