Edited by: Alberto García-Salido - Pediatric Intensive Care Unit, Hospital Infantil Universitario Niño Jesús, Madrid, Spain
Last update: May 2024
More infoTo identify early prognostic factors that lead to an increased risk of unfavorable prognosis.
DesignObservational cohort study from October 2002 to October 2017.
Setting and patientsPatients with severe TBI admitted to intensive care were included.
Variables and interventionsEpidemiological, clinical, analytical and therapeutic variables were collected. The functional capacity of the patient was assessed at 6 months using the Glasgow Outcome Scale (GOS). An unfavorable prognosis was considered a GOS less than or equal to 3. A univariate analysis was performed to compare the groups with good and bad prognosis and their relationship with the different variables. A multivariate analysis was performed to predict the patient's prognosis.
Results98 patients were included, 61.2% males, median age 6.4 years (IQR 2.49–11.23). 84.7% were treated by the out-of-hospital emergency services. At 6 months, 51% presented satisfactory recovery, 26.5% moderate sequelae, 6.1% severe sequelae, and 2% vegetative state. 14.3% died. Statistical significance was found between the score on the prehospital Glasgow coma scale, pupillary reactivity, arterial hypotension, hypoxia, certain analytical and radiological alterations, such as compression of the basal cisterns, with an unfavorable prognosis. The multivariate analysis showed that it is possible to make predictive models of the evolution of the patients.
Conclusionsit is possible to identify prognostic factors of poor evolution in the first 24 h after trauma. Knowledge of them can help clinical decision-making as well as offer better information to families.
Identificar factores pronósticos precoces que conduzcan a un mayor riesgo de pronóstico desfavorable.
DiseñoEstudio de cohortes observacional de octubre 2002 a octubre 2017.
Pacientes y ámbitoSe incluyeron pacientes menores de 18 años con TCE grave ingresados en cuidados intensivos (UCIP).
Variables e intervencionesSe recogieron variables epidemiológicas, clínico-analíticas y terapéuticas. Se valoró la capacidad funcional del paciente a los 6 meses mediante la Glasgow Outcome Scale (GOS). Se consideró pronóstico desfavorable un GOS menor o igual a 3. Se realizó un análisis univariante para comparar grupos de buen y mal pronóstico y su relación con las diferentes variables. Se realizó un análisis multivariante para predecir el pronóstico del paciente.
Resultados98 pacientes, 61,2% varones, mediana de edad 6,4 años (RIQ 2.49–11.23). El 84,7% fueron atendidos por los servicios de emergencias extrahospitalarios. A los 6 meses, el 51% presentaba recuperación satisfactoria, 26,5% secuelas moderadas, 6,1% secuelas graves y 2% estado vegetativo. Fallecieron el 14,3%. Hubo significación estadística entre la puntuación en la escala de coma de Glasgow (ECG) prehospitalaria, reactividad pupilar, hipotensión arterial, hipoxia, ciertas alteraciones analíticas y radiológicas (compresión de las cisternas basales), con pronóstico desfavorable. El análisis multivariante demostró que es posible realizar modelos predictores de la evolución de los pacientes.
ConclusionesEs posible identificar factores pronósticos de mala evolución en las primeras 24 horas postraumatismo. Su conocimiento puede ayudar a la toma de decisiones clínicas y ofrecer una mejor información a las familias.
Severe traumatic brain injury (TBI) is the main cause of death and disability in children over one year of age in developed countries1. Almost 98% of all such cases are of mild severity1; as a result, the studies on moderate to severe TBI are limited and difficult to carry out, particularly considering the lack of registries referred to patients with TBI. The epidemiology of severe pediatric TBI in Spain has recently been described by Cabrero-Hernandez et al.2. Most of the knowledge about severe TBI in children, its prognostic factors and its consequences have been extrapolated from studies in adults. However, there are important differences between the brain of the child and that of the adult in terms of the pathophysiological response to injury, recovery capacity, and plasticity. Assessment of the factors that influence the prognosis is difficult, since many clinical, radiological and laboratory test parameters are involved, in addition to factors related to the patient, the injury, complications and posterior measures and interventions.
In the first 24 h following trauma, several prognostic factors have been correlated to increased morbidity-mortality in children with severe TBI3. Knowledge of these factors can help to identify patients with an increased risk of death or of presenting an unfavorable neurological outcome.
Material and methodsAn observational cohort study was carried out covering the period from October 2002 to October 2017. Part of the information was collected on a prospective basis from a polytrauma patient registry, while other data were compiled retrospectively (review of case histories of the Pediatric Intensive Care Unit [PICU], hospitalization area and clinics).
We included the patients with severe TBI (Glasgow Coma Scale (GCS) < 9) admitted to the PICU during the study period. To classify TBI as severe, we assessed the prehospital GCS or the GCS in the hospital of origin of the patient, and in the first four hours following admission to the study hospital.
Patients with incomplete data were excluded, as were those in which assessment of the neurological prognosis was not possible (because of a previous disease or loss to follow-up), patients that died during the first hours following admission in which the existence of TBI could not be confirmed (no brain computed tomography [CT] scan made), and those that died secondary to extracranial trauma.
The following variables were recorded: epidemiological data, mechanism of injury, prehospital clinical evaluation (GCS, pupils, seizures, arterial hypotension, hypoxia), prehospital treatment (intubation, administered fluids and drugs, cardiopulmonary resuscitation [CPR]), hospital clinical evaluation (GCS, pupils, seizures, arterial hypotension, hypoxia, vital signs, intracranial pressure [ICP]), laboratory test data (glucose, pH, pCO2, HCO3, hemoglobin, platelets, electrolytes and coagulation), radiological parameters, hospital treatment (mechanical ventilation [MV], fluids, blood products, neurosurgery, sedoanalgesia, anticonvulsants, hyperosmolar therapy, barbiturate coma, craniectomy) and associated extracranial lesions.
We evaluated patient functional capacity at discharge from the PICU and at 6 months post-injury based on the Glasgow Outcome Scale (GOS), and reviewed the case histories and patient follow-up in the clinics of different specialties.
A favorable prognosis was defined as a GOS score of 4 (moderate sequelae) or 5 (satisfactory recovery), while an unfavorable prognosis was defined as a GOS score of 3 (serious sequelae), 2 (vegetative state) or 1 (deceased).
Statistical analysisQuantitative variables were reported as the median and interquartile range (IQR), while qualitative variables were reported as absolute (n) and relative frequencies (%).
A univariate analysis was made to compare the groups with a good and a poor prognosis, and their association to the rest of the variables. The distribution of quantitative variables was compared using the Mann–Whitney U-test, while qualitative variables were compared with the chi-square test.
Multivariate logistic regression analysis was performed to predict the patient prognosis, using as predictors those variables found to exhibit the greatest predictive value in the univariate analysis: prehospital GCS, pupillary response upon admission, hypoxia or arterial hypotension, and compression of the basal cisterns. Concerning the prehospital GCS, we sought the cut-off point with the greatest capacity to distinguish between patients with a good and a poor prognosis. Models with three predictive variables were adjusted. To measure the predictive capacity of the model, we estimated the area under the receiver operating characteristic (ROC) curve of the predicted probabilities, and compared the models based on the index obtained.
The study was approved by the Clinical Research Ethics Committee of Hospital Infantil Universitario Niño Jesús (Madrid, Spain).
ResultsDuring the study period, a total of 531 polytraumatized patients were attended, of which 382 suffered TBI (103 with severe TBI). Five arrived in hospital in critical condition and died within the first three hours of admission, without being able to clarify the cause of death, and were excluded from the study. A total of 98 patients were thus analyzed.
A total of 61.2% were males. The median age upon admission was 6.4 years (IQR 2.49–11.23). The prognosis was analyzed by age group: under two years of age (19/98), 2–8 years of age (40/98) and over 8 years of age (39/98). Neither patient gender nor age group conditioned a greater frequency of unfavorable outcomes.
The main mechanisms of injury were falls (40.8%), followed by traffic accidents (32.7%). No relationship was observed between the mechanism of injury and a favorable/poor prognosis.
Most of the patients (84.7%; 83/98) were attended by the out-hospital emergency services at the site of the accident. In 76.5% of the cases, patient transfer was by means of the mobile intensive care unit. Of the patients taken to hospital in private vehicles (15/98), 26% had an unfavorable prognosis (4/15), versus 21.6% of the patients attended by the out-hospital emergency services (18/83).
Clinical variablesThe median prehospital GCS was 7 (IQR 4–8.75) and was significantly lower in the patients with a poor prognosis (p < 0.05). We assessed the cut-off point with the greatest capacity to distinguish between patients with a favorable or an unfavorable prognosis based on the calculation of the sensitivity and specificity of the GCS scores and the plotting of ROC curves. The optimum point was found to be 5, with a sensitivity of 86.67%, a specificity of 71.43%, and an area under the ROC curve of 0.847 (95% confidence interval [95%CI]: 0.74−0.96). A total of 31.6% of the patients (31/98) had a score of ≤ 5. Of these, 58% (18/31) presented an unfavorable course, versus 5.9% (4/67) of those with a score of > 5 points. In turn, 81.8% of the patients (18/22) with a poor prognosis presented GCS ≤5. Upon admission to the PICU, the GCS was found to be significantly lower than the prehospital GCS (5.58 [IQR 3–7)] – this being related to the fact that 84.7% of the cases (83/98) had received sedation in the context of initial stabilization.
In the prehospital pupillary exploration, 72.4% of the patients (71/98) presented normal pupillary reaction, while 14.3% (14/98) had a non-reactive pupil and 13.3% (13/98) presented non-reactivity of both pupils. A significant association was found between an altered pupillary exploration and an unfavorable prognosis (p < 0.05).
At prehospital assessment, 28.6% of the patients presented arterial hypotension, 33.7% hypoxia, and 11.2% underwent cardiopulmonary resuscitation (CPR) maneuvers – the latter patients presenting an unfavorable prognosis (p < 0.05) (Table 1). During the first 24 h of admission to the PICU, 22.4% of the patients presented arterial hypotension and 16% hypoxia. Cardiopulmonary resuscitation was performed in 10.2% of the cases.
Prehospital arterial hypotension, hypoxia and CPR, and patient prognosis.
| Total n = 98 (%) | Good prognosis (n = 76) | Poor prognosis (n = 22) | p | |
|---|---|---|---|---|
| Hypotension | ||||
| No | 70.2% | 83.6% | 23.8% | 0.00 |
| Yes | 29.8% | 16.4% | 76.2% | |
| Hypoxia | ||||
| No | 64.5% | 76.4% | 23.8% | 0.00 |
| Yes | 35.5% | 23.6% | 76.2% | |
| CPR | ||||
| No | 88.8% | 98.7% | 54.6% | 0.00 |
| Yes | 11.2% | 1.32% | 45.4% | |
CPR: cardiopulmonary resuscitation.
A total of 18.4% of the patients suffered seizures, though this was not associated with a poorer outcome (p > 0.05).
Laboratory test parametersLaboratory tests were made in all cases. In the univariate analysis, the presence of acidosis, hypercapnia, lowered bicarbonate, increased lactate, hyperglycemia and the prolongation of cephalin time were significantly associated with a poor prognosis (p < 0.05) (Table 2).
Laboratory test data.
| Good prognosis (n = 76) | Poor prognosis (n = 22) | p | |
|---|---|---|---|
| Median | Median | ||
| pH upon admission | 7.29 (IQR 7.19−7.38) | 7.14 (IQR 7.01−7.26) | 0.00 |
| pCO2 upon admission (mmHg) | 42.5 (IQR 35−53.1) | 51.45 (IQR 35.75−72.2) | 0.04 |
| HCO3 upon admission (mmol/l) | 19.7 (IQR 17−21.9) | 16.65 (IQR 13.45−18) | 0.00 |
| Lactate upon admission (mmol/l) | 2.1 (IQR 1.4−3.6) | 6.6 (IQR 3.5−8.5) | 0.00 |
| Blood glucose upon admission mg/dl) | 143 (IQR 119−172.75) | 227 (IQR 166.2−313.5) | 0.00 |
| Cephalin time upon admission (seconds) | 27 (IQR 23.77−30) | 29.5 (IQR 25.25−41.75) | 0.02 |
HCO3: bicarbonate; n: sample; pCO2: CO2 venous pressure; IQR: interquartile range.
Brain CT was performed in all patients. A total of 76.5% (75/98) suffered an intracranial injury. The presence of subdural hematoma, subarachnoid hemorrhage, ventricular hemorrhage, brain edema and compression of the basal cisterns was significantly associated with a poor prognosis (p < 0.05) (Table 3).
Intracranial injuries and poor prognosis.
| Good prognosis % (n = 76) | Poor prognosis % (n = 22) | p | |
|---|---|---|---|
| Subdural hematoma | |||
| No | 75% (57/76) | 31.8% (7/22) | 0.00 |
| Yes | 25% (19/76) | 68.2% (15/22) | |
| Subarachnoid hemorrhage | |||
| No | 85.5% (65/76) | 54.5% (12/22) | 0.001 |
| Yes | 14.5% (11/76) | 45.5% (10/22) | |
| Ventricular hemorrhage | |||
| No | 97.4% (74/76) | 68.2% (15/22) | 0.00 |
| Yes | 2.6% (2/76) | 31.8% (7/22) | |
| Brain edema | |||
| No | 86.8% (66/76) | 36.4% (8/22) | 0.00 |
| Yes | 13.2% (10/76) | 63.6% (14/22) | |
| Compression of the basal cisterns | |||
| No | 84.2% (64/76) | 45.5% (10/22) | 0.00 |
| Yes | 15.8% (12/76) | 54.5% (12/22) | |
| Epidural hematoma | |||
| No | 88.18% (67/76) | 90.91% (20/22) | 0.719 |
| Yes | 11.84% (9/76) | 9.09% (2/22) | |
| Brain contusion | |||
| No | 57.89% (44/76) | 50% (11/22) | 0.511 |
| Yes | 42.11% (32/76) | 50% (11/22) | |
| Diffuse axonal injury | |||
| No | 86.84% (66/76) | 77.27% (17/22) | 0.272 |
| Yes | 13.16% (10/76) | 22.73% (5/22) | |
| Midline deviation | |||
| No | 77.63% (59/76) | 81.82% (18/22) | 0.246 |
| Yes | 22.37% (17/76) | 18.18% (4/22) | |
All patients received crystalloids in initial care. A total of 58.2% required the transfusion of packed red cells in the first 24 h, 38.8% frozen fresh plasma, 8.2% platelets and 10.2% coagulation factors. Inotropic drug support proved necessary in 57 patients (58.2%). Invasive mechanical ventilation was used in 99% of the cases (97/98). Intracranial pressure (ICP) monitoring was performed in 41 patients (41.8%), with a greater frequency in patients with GCS ≤5 and altered pupillary response; intracranial hypertension (ICHT) was present in 32 of the cases. On the other hand, 41.4% of the patients (17/41) subjected to ICP monitoring had a poor prognosis, versus 8.7% of those (5/57) in which no such monitoring was made. A total of 25.5% of the patients (25/98) required urgent neurosurgery. The intracranial lesions that most often required surgery were epidural (63.6%, 7/11) and subdural hematoma (32.3%, 11/34). In turn, 68% of those operated upon presented midline deviation. Decompressive craniectomy was performed in 13.3% of the cases (13/98). A total of 79% of the operated patients had a favorable prognosis.
PrognosisBased on the GOS score at discharge from the PICU, 42.9% of the patients (42/98) presented satisfactory recovery, 21.4% (21/98) had moderate sequelae, 16.3% (16/98) had serious sequelae, and 5.1% (5/98) were in a vegetative state. A total of 14.3% of the patients (14/98) died. Based on the GOS at 6 months, 51% (50/98) presented satisfactory recovery, 26.5% (26/98) had moderate sequelae, 6.1% (6/98) had serious sequelae, and 2% (2/98) were in a vegetative state.
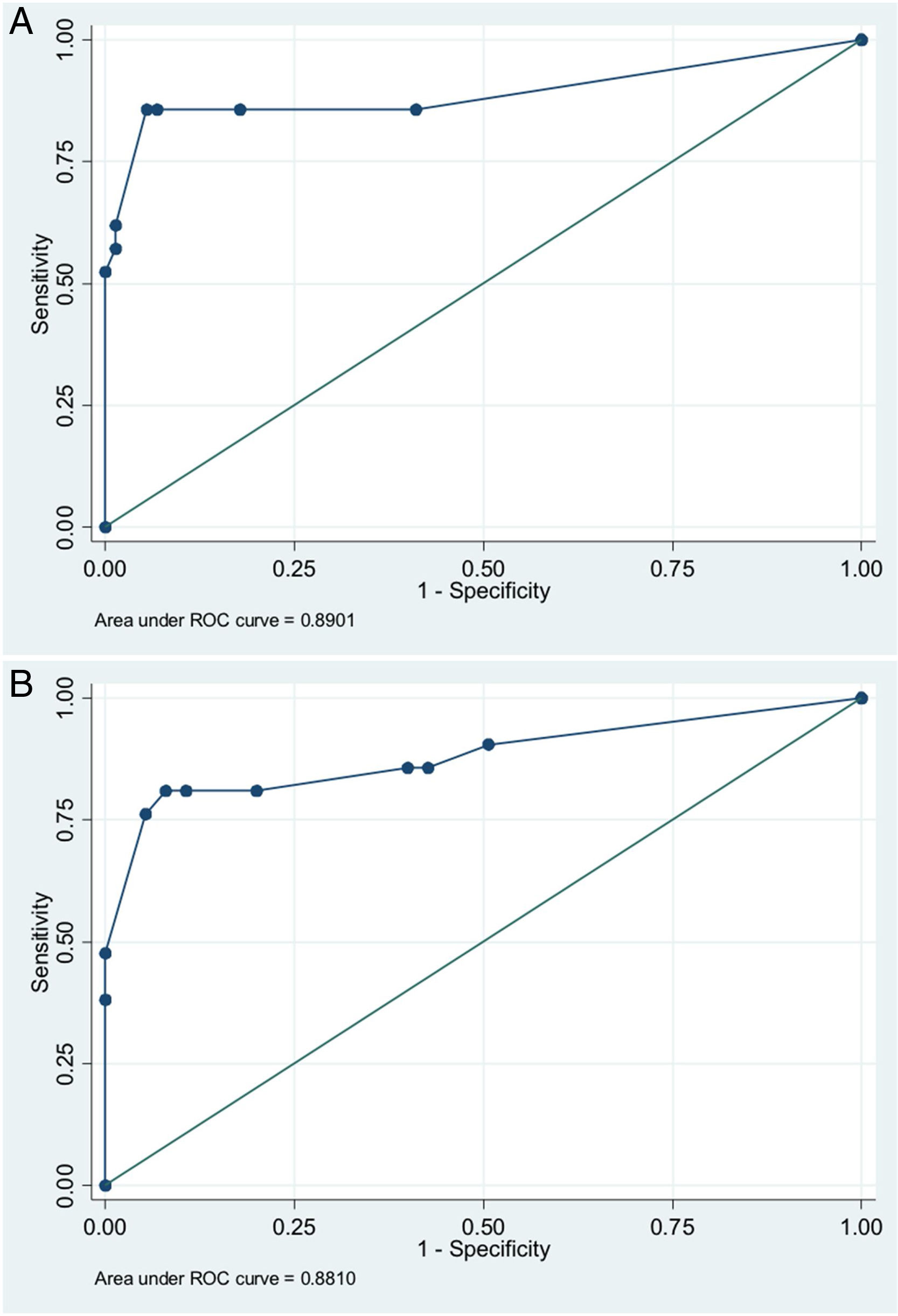
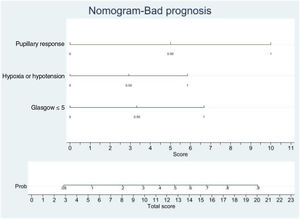
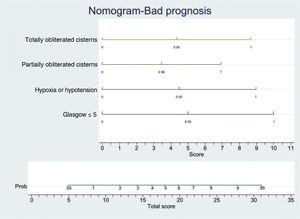
In order to establish a prognostic model and determine the combination of factors best able to predict an unfavorable outcome, we examined prehospital GCS (≤5), pupillary response, compression of the basal cisterns, hypoxia and arterial hypotension, as these were the variables with the greatest predictive value. Models were adjusted with three predictive variables. We analyzed two models that presented a similar predictive capacity (area under the ROC curve). Model 1 (area under the ROC curve = 0.8901; Fig. 1A) was based on prehospital GCS ≤5 (odds ratio [OR]: 7.3; 95%CI: 1.62–3.28), the absence of pupillary response (OR: 19.71; 95%CI: 2.88–134.87) and hypoxia/hypotension (OR: 5.71; 95%CI: 1.22–2.68). Model 2 (area under the ROC curve = 0.881; Fig. 1B) in turn was based on prehospital GCS ≤5 (OR: 9.53; 95%CI: 2.11–430.7), hypoxia/hypotension (OR: 7.52; 95%CI: 1.72–32.80) and compression of the basal cisterns (partially obliterated [OR: 4.76; 95%CI: 0.61–370.6] or totally obliterated [OR: 70.5; 95%CI: 1.12–44.60]).
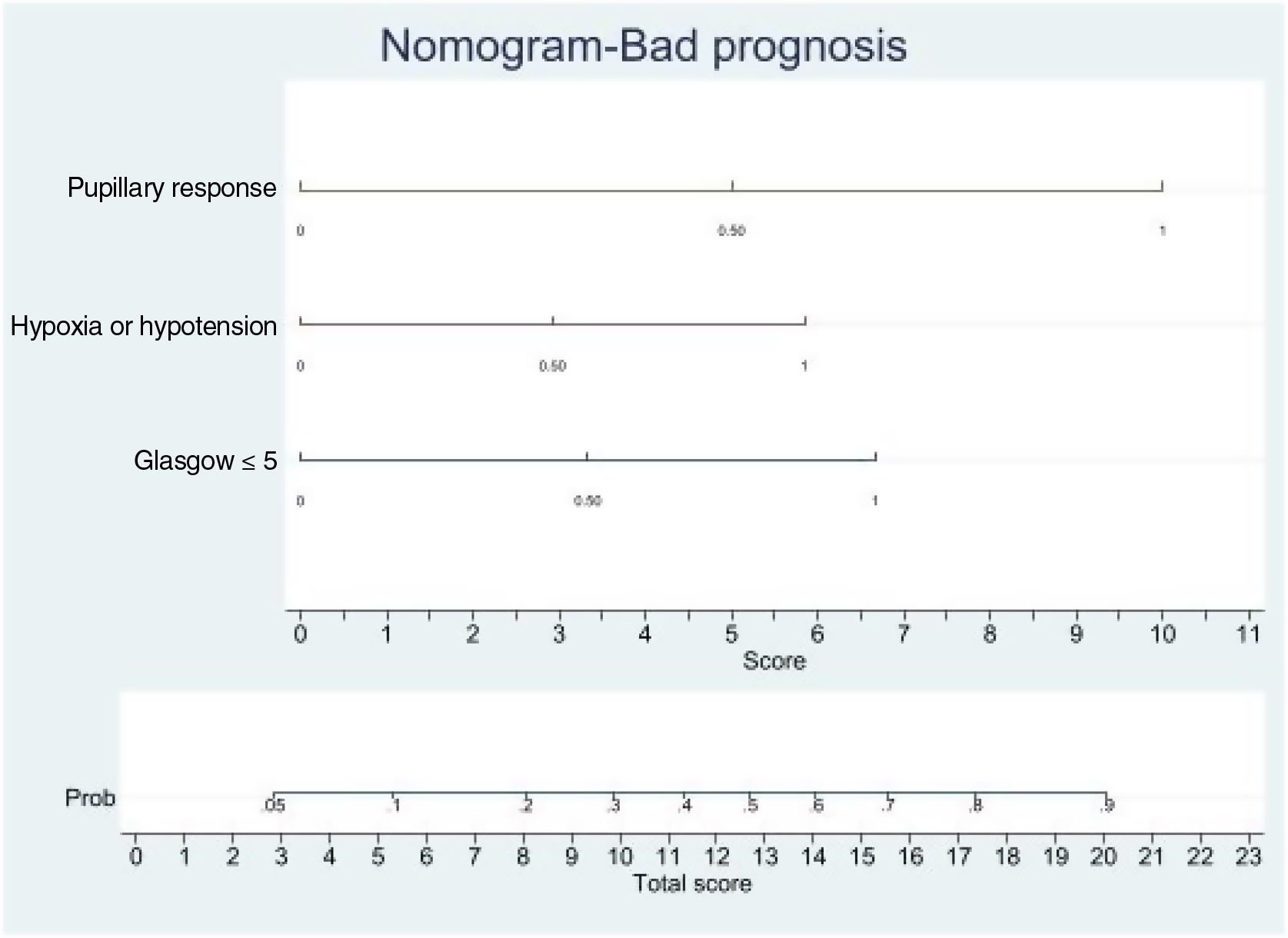
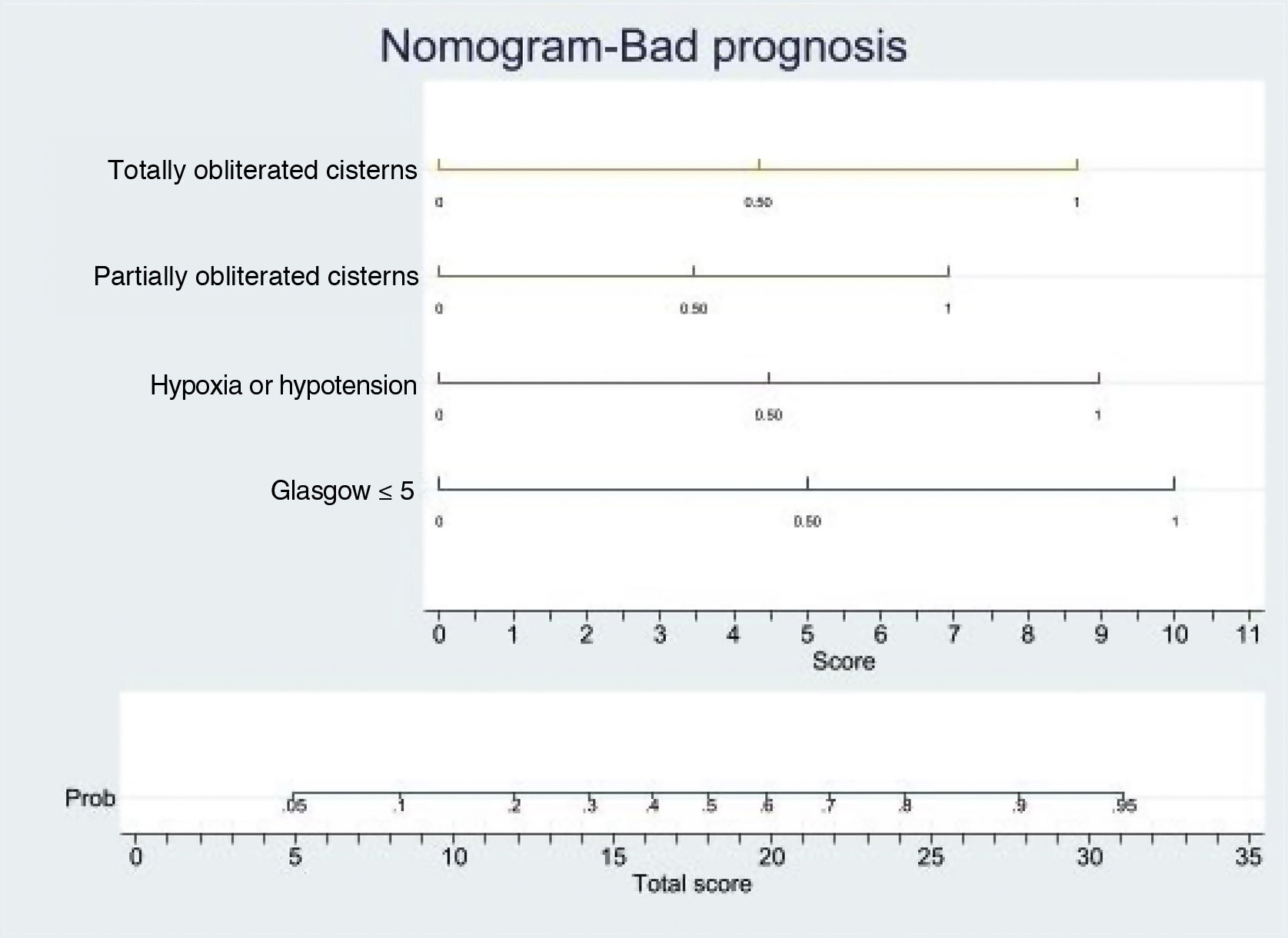
Logistic regression analysis yielded a nomogram in each model, where each factor produced a score on a scale predicting a poor prognosis at 6 months (Figs. 2 and 3).
Nomogram. Poor prognosis scale. Predictive variables: GCS ≤5, absence of pupillary response and hypoxia/arterial hypotension.
The presence of the three factors confers a probability of a poor prognosis at 6 months of > 90%. The sum of GCS ≤5 and the absence of pupillary response implies a risk of a poor prognosis of 75%, while GCS ≤5 and hypoxia/hypotension imply a risk of 50%. The absence of pupillary response and hypoxia/hypotension imply a risk of 70%. In this model, the absence of pupillary response is the strongest indicator of a poor prognosis.
Nomogram. Poor prognosis scale. Predictive variables: GCS ≤5, hypoxia/hypotension and compression of the basal cisterns.
The presence of GCS ≤5, totally obliterated basal cisterns and hypoxia/hypotension confer a probability of a poor prognosis at 6 months of 90%, while GCS ≤5 and totally obliterated basal cisterns imply a risk of 55%. In turn, GCS ≤5 and hypoxia/hypotension confer a risk of 55%. In this model, GCS ≤5 is the strongest indicator of a poor prognosis.
Severe TBI in children has a strong impact on society. The present study has increased our knowledge of this problem, differentiating it from that found in adults. Valuable information has been obtained, allowing us to assess the clinical outcomes and draw conclusions based on the characteristics of our population and our experience.
Many studies have shown the GCS score to be the most important prognostic factor in severe TBI4–6. Thus, the first neurological assessment is crucial for determining the posterior evolution and treatment. Measurement of the GCS at both the site of the accident and upon arrival in hospital is of prognostic value. Although some studies4,5 point to GCS upon admission as the best predictor, our findings suggest the prehospital GCS score to be more useful. This is because almost all the patients reached the PICU after having been stabilized and intubated by the out-hospital emergency services, with the administration of sedation, which can alter the score obtained upon admission in a significant number of patients – thus causing the scale to lose predictive value.
In relation to the GCS score, we explored the cut-off point with the best capacity to distinguish between patients with a favorable and an unfavorable prognosis. This critical score was found to be ≤5, and seems to be the most useful option for identifying a subgroup of patients with greater clinical severity and a greater probability of experiencing a poor outcome. This result is consistent with the data obtained in the study carried out by Chung et al.6, who likewise suggested that this should be the cut-off point for classifying TBI as being serious in children. On the other hand, recent research has described a series of clinical-radiological factors associated with early brain death in adults with intracranial lesions, including GCS < 57.
Approximately one of every four of our patients presented an altered pupillary response. Likewise, a number of them presented arterial hypotension, hypoxemia and required CPR. According to the current evidence, these circumstances have a strong impact on the outcome of patients with TBI, and are the most deleterious systemic factors - contributing to the increase in morbidity-mortality. Arterial hypotension was more frequent during the first 24 h of admission than during prehospital management, and both circumstances were significantly associated with a poor clinical course, in coincidence with the observations of other authors8,9. In contrast, hypoxemia was more frequent during the initial stabilization phase than during the first 24 h of admission – and both of these circumstances likewise were correlated to a poor prognosis. In 1993, Pigula et al.10 reported that arterial hypotension or hypoxia upon admission multiplied the mortality risk four-fold. Treating these conditions as early as possible is therefore essential to improve the prognosis. These data underscore the importance of good out-hospital management and of starting on-site resuscitation as quickly as possible. This is particularly so in the pediatric patient, where initial airway stabilization and the maintenance of adequate oxygenation are decisive. All the patients who suffered cardiorespiratory arrest shortly after trauma had an unfavorable prognosis. This is consistent with the data found in the literature11.
Seizures were not infrequent, and most occurred immediately after injury. The incidence of immediate seizures was higher than reported in the literature12, probably due to the large number of patients attended by the out-hospital emergency services, which allowed due identification of such events. As in other studies, no correlation was found to either prognosis or the mortality rate13.
Altered laboratory test parameters are frequent in children with severe TBI. Although there is an association between certain parameters and the patient prognosis, it is not clear whether the rapid correction of such alterations can modify the clinical outcome. In our patients, the laboratory test parameter most closely correlated to a poor clinical course was hyperglycemia. Other authors14,15 likewise have found hyperglycemia upon admission or during the first 24 h to be associated with a poorer prognosis. The incidence of coagulation disorders was high, particularly decreased prothrombin time and cephalin time — in coincidence with the observations of other investigators16.
The radiological alterations identified by the first brain CT scan are important for determining the seriousness of the injury and the neurological prognosis of the patient17. In the adult population, up to 90–93% of all patients present intracranial damage visible on the first brain CT scan18,19. In our series, this percentage was lower (76.5%), but similar to the figures reported by other studies in pediatric patients20,21. The most frequently identified alterations were parenchymal contusion, followed by subdural hematoma (SDH) and epidural hematoma (EDH), with a distribution very similar to that described in both adults and children19,20. Only SDH was associated with a poorer prognosis. We recorded radiological signs of ICHT, such as compression of the basal cisterns or brain edema, both of which are indicative of greater severity of the injury, in the same way as is seen in adult patients, where compression or absence of the basal cisterns increases mortality. In contrast, midline deviation in children was not associated with greater morbidity-mortality19,22. This could be because almost 70% of the operated patients presented midline deviation, and surgery therefore would have improved the prognosis of this type of lesion.
According to the management guides of the Brain Trauma Foundation23, the monitoring of ICP would be indicated in children with abnormal CT findings and in some patients with normal CT findings but with other factors indicative of a poor outcome. In our series, ICP was monitored in 42% of the patients. A total of 75% developed ICHT, versus only one-half in other publications24,25. This suggests that ICP was monitored in patients correctly identified as being at high risk of presenting ICP elevation. The measurement of ICP was more often carried out in patients with GCS ≤5 and altered pupillary response, as well as in patients with SDH, brain contusion, subarachnoid hemorrhage (SAH), brain edema and compression of the basal cisterns as evidenced by the CT study. Forty-one percent of the patients subjected to the monitoring of ICP had a poor prognosis, versus 8.7% of those in which ICP was not monitored.
The treatment of patients with severe TBI is based on the guides of the Brain Trauma Foundation22,23. Adherence to these guides has been shown to reduce mortality and improve the prognosis26,27. In our series, 85% of the children were stabilized and intubated before arriving in the PICU. The literature reports broad variations in the frequency of intubation of these patients, and this appears to depend on the country and on the degree of development of their out-hospital management systems. In this regard, the figure recorded in our study is higher than in most publications to date28–30. Approximately 60% of the patients required the transfusion of packed red cells and inotropic support — an association being observed between both of these measures and an unfavorable course. In turn, 25% of the patients required urgent neurosurgery — this percentage being similar to the figures reported by other studies (12–30%)31.
In developed countries, TBI is the main cause of mortality in children over one year of age and constitutes an important cause of sequelae32. In our study, the mortality rate was (14%), similar to that found in other studies with similar pediatric patient care systems21,33. The factors that may have contributed to the low mortality in our series are the presence of an advanced out-hospital management system specifically prepared for dealing with patients of this kind; the location of the Unit in the central urban setting, resulting in generally short patient transfer times; and the improvement of hospital management through the creation of the polytraumatized patient care unit.
Of the patients included in the study, 76% presented a favorable prognosis at 6 months. In the literature, this percentage ranges widely from 50 to 90%20,21. One-half of the children presented satisfactory recovery.
The multivariate analysis, based on three clinical or radiological parameters easily obtained in all the patients with severe TBI, showed that the patient prognosis can be established with considerable precision. The predictive model with the best area under the ROC curve was that which included a prehospital GCS score of ≤5, pupillary response and the presence of hypoxia/hypotension as predictors. Likewise, a second model was developed with good predictive capacity using the variables prehospital GCS ≤5, hypoxia/hypotension and compression of the basal cisterns. These models are not intended to replace clinical judgment, but offer a rapid estimate of the probability of a poor patient course, and this may prove useful for clinical management and due information of the families of the patients.
This study has the limitations inherent to its single-center observational nature. The data were obtained from multiple sources, with the possibility of losing some information or of defects in interpretation — though most of the study variables were objective and easy to measure, thereby reducing the possibility of error. Likewise, part of the information was collected on a retrospective basis. On the other hand, the few patients with an unfavorable prognosis limited the multivariate analysis of the morbidity-mortality predictive factors.
It can be concluded that within the first 24 h following trauma, it is possible to identify those children with severe TBI that are at an increased risk of death or of suffering serious sequelae over the long term. A prehospital GCS score of ≤5, altered pupillary response upon admission, the presence of arterial hypotension or of hypoxemia during the first 24 h, as well as compression of the basal cisterns, identify those patients more likely to present a poor prognosis. Knowledge of these prognostic factors may help in clinical decision making, offer better information for the families, and adequately classify patients for future research projects.
Financial supportThe present study has received no financial support.
AuthorshipAll the authors are responsible for the investigation, and all of them have participated in the conception and design of the study, data analysis and interpretation, drafting and correction of the manuscript, and approval of the final text submitted for publication.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Cabrero Hernández M, Iglesias Bouzas MI, Martínez de Azagra Garde A, Pérez Suárez E, Serrano González A, Jiménez García R. Factores pronósticos precoces de morbimortalidad en el traumatismo craneoencefálico grave en niños. Experiencia en una unidad de politraumatismo infantil. Med Intensiva. 2022;46:297–304.