Postcardiotomy cardiogenic shock represents the most serious expression of low cardiac output syndrome after cardiac surgery. Although infrequent, it is a relevant condition due to its specific and complex pathophysiology and important morbidity-mortality. The diagnosis requires a high index of suspicion and multimodal hemodynamic monitoring, where echocardiography and the pulmonary arterial catheter play a main role. Early and multidisciplinary management should focus on the management of postoperative or mechanical complications and the optimization of determinants of cardiac output through fluid therapy or diuretic treatments, inotropic drugs and vasopressors/vasodilators and, in the absence of a response, early mechanical circulatory support. The aim of this paper is to review and update the pathophysiology, diagnosis and management of postcardiotomy cardiogenic shock.
El shock cardiogénico poscardiotomía representa la situación clínica más grave del síndrome de bajo gasto poscirugía cardiaca. Aunque infrecuente, su fisiopatología específica y compleja y su elevada morbimortalidad lo convierten en una entidad especialmente relevante en el contexto de la medicina intensiva. El diagnóstico requiere un elevado índice de sospecha clínica y monitorización multimodal, con un papel fundamental para la ecocardiografía y el catéter de arteria pulmonar. Su manejo debe ser precoz, escalonado y dinámico, multisistémico, multidisciplinar, basado en resolver potenciales complicaciones mecánicas y optimizar los determinantes del gasto cardiaco mediante aporte de volumen o tratamiento deplectivo, fármacos inotrópicos y vasopresores/vasodilatadores y, en ausencia de respuesta, soporte circulatorio mecánico precoz. El objetivo de este artículo es presentar una revisión narrativa y una actualización de la fisiopatología, el diagnóstico y el manejo clínico del shock poscardiotomía. Además, se proponen pautas de actuación que faciliten el manejo clínico diario.
Postcardiotomy cardiogenic shock (PCS) constitutes the most serious clinical expression of post-heart surgery low cardiac output syndrome (LCOS). It refers to the hemodynamic situation in which cardiac output (CO) is unable to meet the tissue metabolic demands. The disorder manifests as the impossibility of weaning the operated patient from cardiopulmonary bypass, or as a persistent shock after heart surgery, despite the use of vasoactive drugs and/or intraaortic balloon counterpulsation (IAoBC). Postcardiotomy cardiogenic shock is defined by a decrease in CO that leads to hypoperfusion and hypotension, i.e., a cardiac index of <2.0 l/min/m2, systolic blood pressure <90 mmHg (or the need for vasopressors to achieve a systolic blood pressure of ≥90 mmHg), a pulmonary capillary wedge pressure of >16−18 mmHg, and oliguria.1,2 Up to 40% of all postoperative patients with shock present evidence of right ventricular dysfunction at echocardiographic evaluation.
While this clinical situation is relatively infrequent (being observed in up to 6% of all postoperative patients), it is very relevant due to the associated morbidity-mortality (even greater than in other types of cardiogenic shock3), and because it implies increased resource consumption.4,5 In fact, a large percentage of patients require mechanical circulatory support (MCS) measures in order to maintain organ perfusion while contractile function and the functions of the rest of the organs recover.6 However, despite the technological advances, the mortality rates have shown no clear improvement.6 In order to improve the outcomes, it is necessary to adopt a multidisciplinary approach involving expert professionals to identify and treat the condition as early as possible, with the adequate use of MCS systems. This approach would allow us to rescue patients with a good medium to long-term prognosis who otherwise would be unable to survive.7
Post-heart surgery vasoplegic syndrome is intimately related and associated with PCS. Up to 50% of all patients with PCS can develop vasoplegia (low systemic vascular resistance values) with a pathophysiology similar to that of septic shock, and with distributive shock being found in up to 5% of such cases.8 Early identification of the problem is crucial, with the start of vasopressor drug therapy to guarantee organ perfusion.
The present review addresses the pathophysiological concepts related to this clinical condition and proposes management guidelines to help clinicians recognize and adequately treat this difficult and complex situation.
PathophysiologyThree associated main pathophysiological mechanisms can be observed in heart surgery: local surgical damage, ischemia-reperfusion injury (IRI) and extracorporeal circulation (ECC) itself.9
Simplifying the pathophysiology of IRI to make understanding easier, we can define three key elements: massive Ca++ entry to the cell with dysregulation of the homeostatic cellular ionic balance mechanisms; inadequate reperfused oxygen utilization with the formation of free radicals (oxidative stress); and mitochondrial permeability transient pore dysfunction and the inactivation of oxidative phosphorylation. Cardioplegia administered during surgery affords protection by preserving energy after inducing rapid diastolic arrest, slowing the metabolic rate and countering the deleterious effects of IRI using specific protective agents.9 Such myocardial protection depends on intrinsic factors (hypertrophy, functional reserve, etc.)10 and on the capacity of cardioplegia to keep the ion channels that cause energy consumption inactive.11 The imbalance between the damaging and protective mechanisms causes postoperative myocardial stunning and – in its maximum expression – secondary cardiogenic shock.
In addition, ECC triggers an inflammatory response involving different systems: contact, coagulation, fibrinolysis and delayed cellular and humor mechanisms.12,13 The inter-relationship of these systems enhances the consequences of IRI, facilitating organ dysfunction (Supplementary material). Fig. 1E of the Supplementary material provides a schematic representation of the etiopathogenesis of vasodilatory shock. Myocardial stunning and vasoplegia share common causal mechanisms, and a mixed clinical condition is usually observed. However, experimental studies indicate that endothelial recovery takes place at a later stage.14
Initial management and hemodynamic monitoringPostcardiotomy cardiogenic shock needs rapid, dynamic and stepwise management focused on detecting and solving the possible underlying or facilitating causes, and this requires complete and early hemodynamic monitoring of the patient.
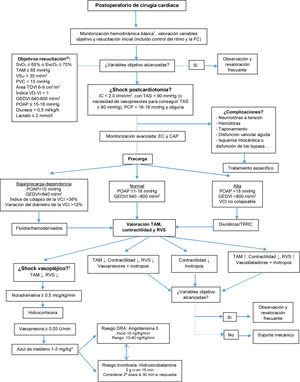
Following admission and basic monitoring, initial optimization guided by hemodynamic objectives is required, following the recommendations of the scientific societies.15–17 Those patients with persistent clinical shock must be subjected to invasive and echocardiographic monitoring to allow due assessment and clarification of the etiology, select the best therapeutic strategy, and monitor the response to the adopted measures18,19 (Fig. 1). In this respect, two monitoring systems are crucial in PCS: echocardiography and the pulmonary artery catheter (PAC).
Monitoring and approach algorithm.
aBasic hemodynamic monitoring: invasive arterial pressure, continuous electrocardiogram, temperature, water balance, mixed or central venous saturation, laboratory testing, blood gases and central venous pressure.
bAdapted from Habicher et al.16
cAdapted from Busse et al.8
dMethylene blue is not recommended in patients with 6-glycophosphate dehydrogenase deficit, selective serotonin reuptake inhibitors (SSRIs), selective noradrenaline-serotonin reuptake inhibitors, monoamine oxidase inhibitors (MAOIs).
PAC: pulmonary artery catheter; AKI: acute kidney injury; EC: echocardiography; HR: heart rate; GLVEDV: global left ventricular end-diastolic volume; CI: cardiac index; PCP: pulmonary capillary wedge pressure; PAOP: pulmonary artery occlusion pressure; CVP: central venous pressure; SVR: systemic vascular resistances; SvcO2: central venous oxygen saturation; SvO2: mixed venous oxygen saturation; MAP: mean arterial pressure; SBP: systolic blood pressure; LVED: left ventricle end-diastolic; CRRT: continuous renal replacement therapy; IVC: inferior vena cava; RV: right ventricle; LV: left ventricle; SVLV: left ventricular systolic volume.
Echocardiography plays the essential role of providing anatomical information quickly and in a noninvasive manner.17 In PCS, the technique should be performed early by expert professionals, allowing the identification of life-threatening complications (Fig. 1) and pericardial effusion, assessment of the function of both ventricles, the estimation of filling pressures, the detection of myocardial ischemia or dysfunction of the aortocoronary grafts (if any), and the estimation of congestion, lung consolidations, effusion or congestion, as well as the exclusion of pneumothorax.20
In turn, PAC allows detailed hemodynamic analysis with the obtainment of information of relevance in PCS — this being the disorder where PAC proves most useful. In effect, PAC is the invasive device of choice in the presence of MCS.21,22 Specifically, PAC can record pulmonary pressures and right and left filling pressures, CO, SvO2 and the ejection fraction and end-diastolic volume of the right ventricle (these latter two parameters can only be obtained with a PAC fitted with a rapid response thermistor).23 Recent studies6,24 point to the importance of PAC in the identification of those patients who require early MCS implantation and those with biventricular failure (this being of help in choosing the device). The catheter also facilitates the optimization of volemia, the adjustment/withdrawal of vasoactive/inotropic drugs, and contributes to guiding patient weaning from MCS. An improved prognosis has even been reported in those patients with cardiogenic shock, especially when MCS is needed.24
In PCS, significant hemodynamic changes take place in short periods of time, requiring close re-evaluation of volemia status. It is just as important to identify those patients who will respond to the administration of fluids with an increase in CO as to identify those with high filling pressures that will require volume depletion therapy. In the presence of volume response parameters associated with high filling pressures or right ventricle failure, a risk/benefit assessment is needed given the possibility of a worsening of the hemodynamic impairment if fluids are administered.
In PCS it is common, particularly in the presence of associated vasoplegic shock, and even when euvolemic conditions are reached, to need to start/adjust vasoactive drugs in order to achieve adequate perfusion pressure. Although the guides recommend a mean arterial pressure of ≥65 mmHg, this target should be individualized according to the basal values of the patient16,19 and the rest of the hemodynamic profile.
Likewise, any alteration of heart rate or rhythm implying significant hemodynamic impairment would require immediate reversal through electrical cardioversion and/or overpacing,17 or revision of the epicardial pacemaker, and the optimization of heart rate.
In the case of left ventricular dysfunction, and after discarding/treating the abovementioned causes, we must analyze contractility and afterload to assess the start/adjustment of inotropic agents, vasoconstrictors or vasodilators.17 If the data referred to hypoperfusion or advanced organ failure persist, we should consider early MCS, since a delay in adopting such measures has been related to increased mortality.6,22
In the presence of right ventricular dysfunction, we must optimize left ventricular function, due to its influence on right-side CO, ensuring adequate ventricular perfusion pressure and reducing right ventricle (RV) afterload by administering pulmonary vasodilators (PVDs). In the absence of a response, MCS should be contemplated.17 This will be addressed more in detail below.
Inotropic agentsThe choice of inotropic drugs in PCS is conditioned by the clinical situation of the patient and the hemodynamic profile. The choice of a specific drug is controversial, due to the lack of randomized multicenter trials involving a large number of patients (attributable to the many difficulties found in carrying out studies of this kind), and is mainly based on clinical experience and on the recommendations and opinions of experts. A Cochrane Library analysis25 of 19 studies involving 2385 patients with cardiogenic shock or low cardiac output syndrome (LCOS)(secondary to infarction, heart failure or post-heart surgery) investigated the efficacy of different inotropic agents: levosimendan versus dobutamine, enoximone or placebo; enoximone versus dobutamine, piroximone or adrenaline-nitroglycerine; adrenaline versus noradrenaline or noradrenaline-dobutamine; dopexamine versus dopamine; milrinone versus dobutamine and dopamine–milrinone versus dopamine–dobutamine. The review concluded that the existing evidence did not allow the recommendation of a specific inotropic drug or combination vasodilator therapy to reduce mortality in patients with cardiogenic shock or LCOS, and underscored the need for high-quality studies to facilitate the choice of drug.
The catecholamines exert positive inotropic and chronotropic effects. Dobutamine and adrenaline improve stroke volume and heart rate, with a moderate decrease in pulmonary capillary wedge pressure and end-diastolic pressure of the left ventricle (LV). Milrinone, a phosphodiesterase 3 inhibitor, increases stroke volume and heart rate, with a decrease in pulmonary capillary wedge pressure and systemic and pulmonary vascular resistances. Levosimendan in turn is a calcium-sensitizing inodilator agent that also increases stroke volume and reduces the vascular resistances. Probably because it is a more novel agent, this drug has been evaluated in a larger number of studies and in consequent reviews and meta-analyses, giving rise to a controversy that persists to date. In patients with LCOS, levosimendan may be indicated as an inodilator, in the early postoperative period; in the case of patients with PCS, it would need to be combined with a vasoconstrictor.
Based on the cumulative experience and on the lack of conclusive studies, dobutamine is usually the first line inotropic agent prescribed. In the event of a poor response and/or chronic treatment with beta-blockers, if the mean blood pressure is >60 mmHg, we may consider milrinone or levosimendan. In the presence of hypotension, it may be necessary to administer a vasoconstrictor such as noradrenaline. In general, adrenaline as an inotropic agent or vasoconstrictor is used as a second-line option.
VasopressorsVasopressors may be classified according to their adrenergic or non-adrenergic actions. Among the adrenergic drugs, the most widely used option is noradrenaline, followed by dopamine and adrenaline.26 Different clinical guides recommend noradrenaline as the first choice in vasodilatory shock27 and in cardiogenic shock, if a vasopressor proves necessary, combined with dobutamine.28 There is no firm evidence to guide the choice of vasopressor drug, and most of the available studies have been carried out in the context of septic shock. However, it can be affirmed that dopamine increases the risk of arrhythmias and possibly of mortality compared with noradrenaline.29 In this respect, noradrenaline offers multiple advantages: a) potency comparable to that of adrenaline and phenylephrine; b) it does not act upon the β2 receptors (lactate may serve as resuscitation guide); c) it does not increase myocardial oxygen consumption; d) it preserves ventricular-arterial coupling, in contrast to adrenaline; and e) it improves CO by increasing the end-diastolic volume (mobilization of splanchnic volume reserve) and through its β1 activity. Nevertheless, all catecholamines favor oxidative stress and interact with cell metabolism and with the inflammatory response. This has led to the development of the “decatecholaminization” concept, seeking to reduce patient exposure to drugs of this kind.
Other non-adrenergic vasopressorsVasopressin and its analogue terlipressin (a prodrug activated by endothelial peptidases to form lysine-vasopressin) act upon the V1a receptors, reducing the production of nitric oxide (NO) and increasing intracellular Ca++ concentration. Studies have been made using vasopressin on a prophylactic basis (0.03 U/min), started before pump activation, resulting in a lesser need for catecholamines and with less postoperative vasodilatory shock.30
In the VANCS trial (<0.04 U/min), the post-heart surgery patients that received vasopressin suffered fewer serious complications than those administered noradrenaline.31 In the VASST trial (septic shock), vasopressin allowed for a decrease in noradrenaline dose, though no differences in terms of mortality were recorded.32
Terlipressin affords similar results, with predominant action upon the V1 receptor, allowing decatecholaminization,33 though there are no conclusive data on the best method of administration (boluses or infusion).
Methylene blue inhibits nitric oxide synthetase and guanylate cyclase — both of which are implicated in the pathophysiology of vasodilatory shock. When used from the intraoperative phase in patients administered angiotensin-converting enzyme inhibitors (ACEIs) up until the day of surgery, it has been associated with a lesser incidence of postoperative vasoplegic shock.34 It is normally used on an off-label basis in cases of refractoriness — usually in the form of “slow” boluses to avoid pulmonary hypertension episodes. Some authors continue with perfusion following the initial bolus dose, observing that early utilization affords greater benefit than as a rescue measure.35
Hydroxocobalamin (vitamin B12) is a potent nitric oxide synthetase inhibitor and binds hydrogen sulfide — an important endogenous vasodilator that interacts with the K-ATP dependent channels. Its first use in heart surgery was as an alternative to methylene blue, in a patient receiving serotonin inhibitors, with good hemodynamic outcomes.36 Posteriorly, there have been case series of refractory shock in which hydroxycobalamine has been used as adjuvant therapy, with promising results,37 including patients subjected to MCS.38 It is not clear when the peak effect and start of action occur, and a degree of inter-individual variability has been reported.37 In the largest case series published to date, the combination of hydroxocobalamin and methylene blue achieved a high percentage of early hemodynamic responses in patients receiving concomitant noradrenaline and vasopressin.39
Corticosteroids reduce the inflammatory response (inhibition of interleukins, cytokines and endotoxins), despite which no decrease in mortality has been observed with their use in cardiac surgery.40 At present, the administration of hydrocortisone as an agent for reducing patient catecholamine exposure is fundamented upon the results of the CORTICUS trial in septic shock41 and on the fact that suppression of the hypothalamic-pituitary-adrenal axis has been observed in ECC.42
Angiotensin II has a dual mechanism of action: it stimulates the adrenal glands, favoring the release of aldosterone and cortisol, and acts upon the sympathetic nervous system and smooth muscle, causing vasoconstriction secondary to intracellular calcium release.43 The ATHOS-3 study demonstrated its capacity to improve the mean blood pressure after three hours of infusion in patients with refractory vasodilatory shock (69.9% versus 23.4%; p < 0.0001; odds ratio [OR] 7.95; 95% confidence interval [95%CI] 4.76–13.3). Of the included patients, 7 required extracorporeal membrane oxygenation (ECMO), and only 6% were postoperative cases.44 In the context of heart surgery, several case series have documented a beneficial effect in reducing the noradrenaline doses.45
With regard to vitamin C and thiamine, recent studies have evidenced that there can be a deficit of this vitamin in critical patients that may be related to hypotension.46 Vitamin C is a cofactor for endogenous amine synthesis,47 and can sensitize the catecholamine receptors, reduce nitric oxide and lower histamine release. A case series has described that the addition of vitamin C may reduce the noradrenaline requirements in the postoperative period of cardiac surgery.46 However, two trials in septic patients, VICTAS (vitamin C, thiamine and hydrocortisone)48 and VITAMINS (vitamin C, hydrocortisone and thiamine versus hydrocortisone),49 revealed no benefits with the triple therapy versus placebo or hydrocortisone alone in reducing the need for amines or the duration of mechanical ventilation.
With regard to the prostaglandin inhibitors, the reduction of prostaglandin production during the inflammatory response could possibly participate in the associated vasoplegia. In a trial in which 18 patients received flurbiprofen, an improved postoperative hemodynamic profile was observed compared with the control group without altered renal function.50
The evidence on adjuvant vasopressors is largely referred to septic patients. In heart surgery, vasopressin is the molecule with the largest body of evidence, after noradrenaline. Consideration is required of the pathophysiology of vasodilatory shock, the timing of administration, and the possible adverse effects when choosing an adjuvant to traditional therapy.
Table 1 describes the characteristics and doses of the inotropic agents and vasopressors most widely used in clinical practice.
Characteristics of the inotropic drugs and vasopressors.
| Drug | Receptors/action site | Recommended dose | Mechanism of action | Side effects |
|---|---|---|---|---|
| Dobutamine | β1, β2 agonist | 1–20 μg/kg/min | Activation of adenyl cyclase, increase levels of intracellular cAMP. ↑ Intracellular Ca++. ↑ Contractility | Arrhythmias |
| Milrinone | PDE3 inhibitor | 0.3–1 μg/kg/min | Inhibits PDE3 enzyme, increases intracellular cAMP. ↑ Intracellular Ca++. ↑ Contractility. | Arterial hypotension, arrhythmia, thrombocytopenia |
| Levosimendan | Calcium sensitizer | 0.05–0.2 μg/kg/min | Enhances Ca sensitivity of contractile proteins and opens ATP-sensitive K channels in vascular smooth muscle. Inodilator | Arterial hypotension, arrhythmia |
| Noradrenaline | α1, α2, β1 agonist | 0.01–1 μg/kg/min | ↑ Intracellular Ca++. Vasoconstriction | High doses: ischemia (intestinal, myocardial, peripheral) |
| Adrenaline | α1, α2, β1, β2 agonist | 0.01−0.5 μg/kg/min | ↑ Intracellular Ca++. Vasoconstriction | High doses: ischemia (intestinal, myocardial, peripheral). Elevation lactic acid |
| Vasopressin | V1–V2 | <0.04 U/min | V1 ↑ intracellular Ca++ (sensitizes catecholamine effect) | High doses: ischemia (intestinal, myocardial, peripheral) |
| Terlipressin | V1a–V2 | 0.5−1 mg boluses/6−8 h | ||
| More selective for V1 receptors | ↓ Synthesis NO | V2 receptors: edema, vasodilatation, microvascular thrombosis (stimulates release of Von Willebrand factor) | ||
| Modulates ATP-dependent K+ channels | ||||
| Vasoconstriction | ||||
| Hydrocortisone | Intracellular cortisol receptor | 50 mg/6 h | Sensitizes catecholamine effect, mineralocorticoid activity. Reduces inflammatory response | Insomnia, nausea, corticosteroid myopathy at high doses, risk of facilitating infections at high doses |
| Methylene blue | Inhibition of guanylate cyclase and iNOS | 1–3 mg/kg bolus | ↓ C-GMP. Vasoconstriction | Hemolysis in 6-GPDH deficit. Serotonin syndrome if SSRI, SSNRI or MAOI. Pulmonary hypertension. Partial heparin inhibitiona |
| 0.5 mg/kg/h during 6–12 h | ||||
| Hydroxocobalamin | Inhibition iNOS | 5 g in 5 min | Modulates ATP-dependent K+ channels | Chromaturia, nausea, headache, hypopotassemia in megaloblastic anemia |
| Hydrogen sulfide binder | ||||
| Angiotensin | AT-R1 | 10–40 ng/kg/min | AT-R1 stimulates SNS: ↑ Intracellular Ca++. Favors release of cortisol and vasopressin. Vasoconstriction AT-R2 vasodilatation | Thromboembolic phenomena, pulmonary hypertension |
| AT-R2 |
cAMP: Cyclic adenosine monophosphate; ATP: adenosine triphosphate; Ca++: ionic calcium; C-GMP: cyclic guanosine monophosphate; MAOI: monoamine oxidase inhibitor; SSNRI: selective serotonin-noradrenaline reuptake inhibitor; SSRI: selective serotonin reuptake inhibitor; NO: nitric oxide; iNOS: inducible nitric oxide synthetase; PDE3: phosphodiesterase 3; SNS: sympathetic nervous system; 6-GPDH: glucose-6-phosphate dehydrogenase.
The clinical scenarios in which right ventricle (RV) failure is observed are coronary disease with right coronary artery damage; valve disorders, particularly of the mitral valve; heart transplantation; congenital heart disease; pulmonary thromboendarterectomy; and patients with left ventricular assist devices.
Postcardiotomy cardiogenic shock secondary to RV failure is characterized by high morbidity-mortality. Management in such situations is similar to that applied in any patient with LCOS and PCS.51 The treatment peculiarities of RV failure (Table 2) include a decrease in RV afterload in order to improve ventricular-arterial coupling, which favors left ventricle (LV) filling and reduces interventricular septum displacement towards the left cavity, improving CO. In addition, situations that produce an increase in pulmonary vascular resistance, such as hypoxemia, hypercapnia and acidosis, must be corrected.52
Management of postcardiotomy cardiogenic shock/right ventricular failure.
| Optimization of preload |
| – Volume supply in case of bleeding or fluid loss. Constant assessment of response to volume |
| – In case of high diastolic blood pressure/water overload: diuretics or renal replacement therapy |
| Optimization of rhythm and heart rate |
| – Preserve sinus rhythm if possible, treatment of tachyarrhythmias |
| – In case of atrioventricular block, use of pacemaker |
| Optimization of ventricular-arterial coupling |
| – Inotropes or inodilator drugs + pulmonary vasodilators i.v. versus inhaled (according to hemodynamic and respiratory condition) (see Table 1E of Supplementary material: vasodilators) |
| – If failure: consider mechanical support (recovery bypass versus transplant) |
| Optimization of perfusion pressure |
| – Use of vasoactive drugs: noradrenaline, vasopressin |
| Optimization of respiratory function |
| – Adjust positive end-expiratory pressure (PEEP). Avoid atelectasis and hyperinsufflation |
| – Consider the use of pulmonary vasodilators to improve respiratory failure |
| – Avoid: hypercapnia, hypoxemia and metabolic acidosis |
| Refractory situations |
| – Persistent shock/mechanical assist pulmonary hypertension (PHT): VA-ECMO |
| – Main problem respiratory failure: VV-ECMO |
In order to reduce RV afterload, use can be made of local (inhaled or nebulized) or systemic (oral/enteral or intravenous) pulmonary vasodilators (PVDs) (Table 1E of Supplementary material).53 The choice of drug substance is conditioned by the clinical situation of the patient.54,55 The use of intravenous PVDs is associated with systemic arterial hypotension, since no strictly selective PVDs are available — a situation that results in poor clinical tolerance in patients with a need for vasopressors. Thus, the inhaled route is the preferred option in these individuals,51,56 being able to largely avoid the deleterious systemic effects. With regard to inhaled PVDs, use is fundamentally made of inhaled nitric oxide (iNO), milrinone, prostaglandins and iloprost. Inhaled nitric oxide is the most widely used drug in relation to this indication. As an advantage, it has a short half-life, while its drawbacks comprise the need for continuous administration, contraindication of the drug once the patient has been weaned from mechanical ventilation, the need to monitor NO2, and possible rebound effects.57 As an alternative, inhaled prostacyclins are widely used in some centers due to the similarity of their effects with respect to iNO.58 Inhaled epoprostenol affords hemodynamic effects very similar to those of iNO, and it also has a short half-life.59 In this context, inhaled prostacyclins such as iloprost offer a more favorable pharmacokinetic profile, with a half-life that allows its administration every 3–4 h, and it can be used in patients subjected to mechanical ventilation or under spontaneous breathing conditions. In hemodynamic terms it is at least as effective as iNO and epoprostenol, being able to reduce the pulmonary artery pressures and improve RV ejection fraction and the cardiac index in a more sustained manner.60,61 At the clinical level, the drug has been shown to improve systemic arterial pressure and reduce the duration of invathe sive mechanical ventilation in patients with RV failure during the immediate postoperative period of heart transplantation. With regard to the potential complications, no differences have been observed in terms of bleeding related to antiplatelet medication or problems related to nebulization.59
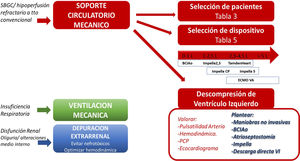
Mechanical circulatory supportIt has been estimated that between 0.3%–3.6% of all cardiac surgery patients present PCS refractory to vasoactive drugs (and in many cases to IAoBC), thus requiring extracorporeal MCS as rescue treatment measure.5,62,63 In the absence of such support, the outcome is potentially fatal, while MCS affords an expectable survival rate of up to 40% — though the figures differ depending on the literature source.63 Adequate selection of the patient, timing of the start of the treatment, the training and experience of the multidisciplinary care team, and the capacity to recognize futility are key factors for securing good clinical outcomes. Table 3 details the indications based on the recent recommendations of various scientific societies. Fig. 2 in turn specifies the mechanical support that may be needed in PCS.
Indications and recommendations regarding mechanical circulatory support in postcardiotomy shock.
| Recommendations | Grade | Level |
|---|---|---|
| It is advisable for support in PCS to start before multiorgan dysfunction or anaerobic metabolism develops (lactate <4 mmol/l) in patients with the possibility of myocardial recovery in the absence of uncontrollable bleeding requiring surgery | I | B |
| When the possibilities of myocardial functional recovery are low, mechanical support is only recommended in patients potentially eligible for heart transplantation or the adoption of long-term mechanical support measures | I | C |
| Early MCS is recommended after heart surgery in patients with IAoBC and optimum medical treatment, with failed weaning from extracorporeal bypass or in situations of severe hemodynamic impairment | I | B |
| Before starting MCS, it is important to assess important comorbidities, advanced age, lactate levels and kidney function, as these are mortality risk factors | IIa | B |
| The type and mode of MCS must be based on the hemodynamic situation and characteristics of the patient: uni- or biventricular failure, right and/or left side failure, pre-/intra-/postoperative circulatory failure, acute or chronic ventricular dysfunction, cardiogenic shock or cardiac arrest | IIa | C |
| ECMO with peripheral cannulation should be considered in patients with PCS, in the presence of left ventricular or biventricular dysfunction | IIa | B |
| In peripheral ECMO with femoral cannulation, the placement of a distal perfusion cannula to reduce the risk of limb ischemia should be considered | IIa | B |
| The oxygenator in right VAD circuit (Oxy-RVAD) configuration should be considered in refractory isolated RV failure | IIb | C |
| In the presence of limb ischemia, despite anterograde perfusion, contralateral femoral, axillary artery or central access should be considered | IIa | C |
| Cannulation of the axillary/subclavian artery or central aortic cannulation should be regarded as an alternative to femoral cannulation, particularly in the context of prolonged support requirements | IIb | C |
| Direct cannulation through the apex of the LV should be considered for LV drainage and for conversion to LV-like assist (apex LV-subclavian artery) | IIb | C |
| The hybrid/alternative configurations (VVA, VAV or others — including additional devices) should be considered in patients with VV- or VA-ECMO with heart failure, Harlequin syndrome (differential hypoxemia), respiratory failure, refractory hypoxemia, insufficient venous drainage and/or LV stasis | IIb | C |
| In certain hemodynamic situations, or in the presence of structural alterations of the heart, other devices should be considered: IAoBC, transaortic or trans-septal devices | IIa | C |
| The placement of IAoBC may be considered in cases of moderate ventricular dysfunction during weaning from bypass before starting MCS, or in the presence of acute heart failure after emerging from bypass, before MSC is started | IIb | C |
| It is not advisable to place IAoBC in cases of severe LV dysfunction or biventricular dysfunction as the first option in patients in which weaning from bypass is not possible or who present acute heart failure following weaning from bypass | III | C |
| The use of IAoBC may be combined with MCS in patients with little or no aortic valve opening on starting MCS with the selected flow | IIb | C |
| The use of a transvalvular microaxial device (percutaneous or axillary) may be considered in PCS as a first option or concomitant to MCS in the presence of isolated LV dysfunction, or in patients with little or no aortic valve opening on starting MCS with the selected flow | IIb | C |
| The short-term use of ventricular assist devices in patients with PCS (isolated RV dysfunction) may be considered as a first treatment option | IIb | C |
| In the presence of signs of LV distension and stasis, closing of the aortic valve and lung edema, it is advisable to adopt conservative measures (adjustment of ECMO flow, vasodilators, use of PEEP), including IAoBC, to facilitate LV unloading. In patients who fail to respond to these measures, the use of other devices (e.g., transaortic systems) is recommended for LV unloading | I | B |
| In the presence of signs of LV distension and stasis, closing of the aortic valve and lung edema, and unloading septostomy may be contemplated | IIb | C |
Levels of evidence: A: data obtained from multiple randomized clinical trials or meta-analyses; B: data obtained from a single randomized clinical trial or large non-randomized studies; C: expert opinion consensus and/or small studies, retrospective studies or registries.
Grades of recommendation: I: evidence and/or general agreement that a treatment affords benefit and is useful and effective. It is recommended/indicated. II: no general agreement on the evidence or there is a divergence of opinion regarding the usefulness/efficacy of a treatment or procedure (IIa: the weight of the evidence/opinion is in favor of usefulness/efficacy. The treatment “should” be considered; IIb: usefulness/efficacy less established by the evidence/opinion. The treatment “may” be considered). III: general agreement that a treatment/procedure is not useful/effective and might even prove to be harmful.
Source: Weman et al.9.
Multisystemic mechanical support in postcardiotomy cardiogenic shock (PCS). PCS should be regarded as a multisystemic disorder requiring the support of multiple organs. In addition to mechanical ventilation and extrarenal filtration techniques, in the case of patients with refractory PCS it is necessary to consider the use of MCS at an early stage. Patient selection is to be carried out according to the data provided in Table 3. The choice of device (Table 1E) is to be made adjusted to the clinical condition of the patient, the need for uni- or biventricular support, lung involvement, greater or lesser flow to achieve adequate organ perfusion, etc. In cases with pulmonary congestion, left ventricular dilatation and/or loss of arterial pulsatility, consideration is required of left ventricular decompression using scantly invasive procedures (adjustment of mechanical support flows, vasoactive drugs and diuretics), or with maneuvers affording greater unloading, such as IAoBC, atrioseptostomy, pulmonary arterial aspiration, the Impella® device (or similar systems), or direct left ventricular unloading.
The timing of MCS implantation has not been well defined, though early implantation should always be considered, either during the surgical procedure in patients that cannot be weaned from ECC, or in the first postoperative hours if shock persists — since delays result in poorer outcomes.64
In general terms, the MCS devices are equipped with a drainage access, a circuit with a centrifugal pump, a return access and, optionally, an interspaced oxygenator. Depending on the configuration of the system, uni- or biventricular support can be provided. The device can involve peripheral or central cannulation and may provide oxygenation support in addition to circulatory support. Axial support systems are also available that are placed in a transvalvular position in either the left or the right cavities. Table 4 shows the main characteristics, advantages and inconveniences of the most commonly used devices.
Characteristics of the mechanical circulatory support systems.
| Percutaneous | Surgical | ||||||
|---|---|---|---|---|---|---|---|
| IAoBC | Tandem heart | Impella® 2,5/5.0/LD/CP/RP | Peripheral VA-ECMO | Central ECMO | Centrifugal VAD | Pneumatic VAD | |
| Mechanism | Intraaortic counterpulsation | Continuous centrifugal flow | Continuous axial flow | Continuous centrifugal flow | Continuous centrifugal flow | Continuous centrifugal flow | Pneumatic pulsatile flow |
| Support | LV | LV/RV according to the configuration | LV (RP → RV) | LV + RV, oxygenation | LV + RV, oxygenation | LV/RV/BiV | LV/RV/BiV |
| LV effect | ↓ LV afterload and work. ↑ CO 0.5 bpm | ↓ LV afterload. ProtekDuo®: RV support | LV unloading | ↑ LV afterload (axillary < femoral) | VA unloading and ↓ work | VA unloading and ↓ work | VA unloading and ↓ work |
| Maximum flow | – | Up to 5 bpm | Impella® 2.5 → 1.8 bpm; CP → 4.3 (3.8); 5.0/LD → 5 bpm (4.3 real) | Cannula diameter dependent (5 bpm approx) | Cannula diameter dependent (5−8 bpm approx) | 9 bpm | 7 bpm |
| Duration | Days | 14 days | 2.5/CP: 4 days; 5.0/LP: 6 days; RP: 14 days | 30 days | 30 days | 30 days | 80 days (mean) |
| Advantages | Rapid insertion, at the point of care | Stability of flows | Hemodynamic profile. Simple implantation | Rapid insertion, at point of care, oxygenation | Stability of flow. Hemodynamic profile | Stability of flow. Hemodynamic profile. ± oxygenator | Durable support. Hemodynamic profile |
| Complications | Embolism, vascular damage, thrombocytopenia | Requires septostomy, post-IAC, displacement cannula, tamponade | Displacement, lesion AoV/ventricle, tamponade, hemolysis, VT, embolia, ischemia | LV thrombosis, ALE, Harlequin syndrome, hemorrhage, limb ischemia | Hemorrhage, embolia | Hemorrhage, embolia | Hemorrhage, CVA |
| Contraindicated | Moderate-severe AoI, aortic dissection, vascular disease | Moderate-severe AoI, aortic dissection, vascular disease | Ventricular thrombus, AoVR (mechanical), AoS < 0.6 cm, moderate-severe AoI, IAC/IVC, moderate-severe LVH | Moderate-severe AoI, aortic dissection, vascular disease, uncontrollable hemorrhage | Aortic dissection, uncontrollable hemorrhage | Anticoagulation not possible | Anticoagulation not possible |
CVA: cerebrovascular accident; BiV: biventricular; IAC/IVC: interatrial/interventricular communication; AoS: aortic stenosis; ALE: acute lung edema; ECMO: extracorporeal membrane oxygenation; LVH: left ventricular hypertrophy; AoI: aortic insufficiency; AoVR: aortic valve replacement; VT: ventricular tachycardia; VA: venous-arterial; VAD: ventricular assist device; AoV: aortic valve; RV: right ventricle; LV: left ventricle.
To date, no studies have compared the different MCS devices on a controlled basis to identify possible significant differences in terms of outcomes and mortality; as a result, the decision as to which device to use should be made on an individualized basis, taking into account factors such as whether failure is uni- or biventricular, whether the patient presents concomitant respiratory failure, the degree and severity of the shock, the possibilities for recovery (or even transplantation options), and the experience and the availability of the teams.65
There is controversy regarding the possible superiority of central cannulation over peripheral cannulation.65 The central approach favors hemodynamic support, with anterograde flow in the aorta and effective unloading of the ventricle, and direct drainage into the atrium allows the generation of greater support flows. In contrast, the peripheral approach allows sternal closure, reducing hemorrhagic and infectious complications. The existing scientific evidence is mainly based on retrospective series. The largest retrospective study to date, and the subsequent meta-analysis, revealed no differences in patient mortality. In contrast, the incidence of bleeding, transfusions and renal failure requiring renal replacement therapy (RRT) was lower in the peripheral cannulation group.64,65 On the other hand, a study and posterior meta-analysis did record better survival among the patients subjected to peripheral venoarterial extracorporeal membrane oxygenation (VA-ECMO).66 The complications, particularly in arterial cannulation, are of a hemorrhagic and ischemic nature in the affected extremity. There are return alternatives, such as the axillary artery-subclavian approach, which returns anterograde flow and therefore increases afterload to a lesser degree than the femoral access, without having to perform a sternotomy.67 Cannulation of the pulmonary artery can be used for return in right-side assist or as drainage to drain the right and left cavities.68
A relatively frequent complication when peripheral VA-ECMO is used is an increase in LV afterload generated by the retrograde flow of the system, which implies that the native myocardial function is unable to generate systolic volume, and the aortic valve may even fail to open correctly. This generates lung congestion and an increase in intraventricular pressure — reducing the possibilities for the recovery of myocardial function. In the initial phase of ventricular distension, we must provide conservative management based on the reduction of MCS flow, the administration of inotropic agents and the use of vasodilators. These measures, either alone or associated to IAoBC, may be enough. If this were no the case, however, or if severe lung congestion develops, we should consider direct drainage of the left cavities using a trans-aortic system, an interatrial catheter, or direct drainage of the left cavities via a surgical approach.69
Although VA-ECMO is the most widely used type of support in PCS (especially via the peripheral route with femoral accesses),70 there are also alternative and/or complementary supports. In this respect, IAoBC is still often the first adopted support measure, since it is easy to implant, effective and has a low incidence of complications.71,72 The Impella® range of systems generates effective univentricular support. The experience gained in patients with PCS is limited, though it may be an adequate choice in selected subjects without severe respiratory failure and under non-urgent clinical conditions. The use of short-duration ventricular assist measures may be appropriate as a first option in selected cases, to provide uni- or biventricular support, adopting an interspaced oxygenator if necessary.
Acute renal dysfunction associated with PCS (Supplementary material)The appearance of acute renal dysfunction in heart surgery is associated with a mortality rate of 22% — a figure that reaches up to 65% when RRT is required. Of the survivors, approximately 10% do not recover renal function and subsequently become dependent upon hemodialysis.73 In patients with PCS, acute renal dysfunction may prove serious and persist for weeks or months.74 At present, and after many trials and meta-analyses,75–80 there is no firm evidence to suggest that the early start of RRT reduces mortality. A conservative strategy for starting RRT following heart surgery, with well-defined objectives, is an acceptable approach that can allow many patients to recover renal function without the potential complications of the technique.
In sum, it can be concluded that PCS is a very relevant condition in the context of intensive care medicine and critical care, with high morbidity-mortality. Adequate knowledge of the disorder and its rapid and protocolized management, based on full monitoring and experience, with the early use of MCS (if needed), may improve the patient course and outcome. Management guidelines are provided that may be useful for clinicians who need to deal with patients of this kind in their daily practice.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Pérez Vela JL, Llanos Jorge C, Duerto Álvarez J, Jiménez Rivera JJ. Manejo clínico del shock poscardiotomía en pacientes adultos. Med Intensiva. 2022;46:312–325.