The use of plasma from patients who have recovered from viral infections was used for the first time back in the 20th century to treat cases of the so-called Spanish flu1. More recently, plasma from convalescent patients (PCP) has been effective and safely used to treat respiratory infections due to SARS-CoV and the Middle East respiratory syndrome (MERS-CoV)2. Some studies have demonstrated a better clinical evolution when used within the first 14 days3. Back in 2014, the World Health Organization (WHO) recommended its use to treat Ebola virus disease categorizing it as empirical treatment4. One year later, several agencies were recommending PCP as one of the potential therapies to treat severe cases of MERS-CoV5.
The SARS-CoV-2 pandemic caused a health crisis worldwide collapsing intensive care units (ICU) with patients with pneumonia and acute respiratory distress syndrome (ARDS). Because of the lack of effective treatments, the use of plasma from convalescent patients was initially suggested as a source of antibodies with the idea that it could improve the course of the disease if used early by reducing early viremia for the lack of antibodies or when the total count of antibody titers (IgM and IgG) is low6,7.
A study was conducted with patients admitted to a polyvalent ICU at a tertiary care hospital with 44 beds prior to the pandemic (from September 2020 through December 2020) to assess the efficacy and safety profile of PCP in severe patients with COVID-19 who had not developed antibodies. To assess efficacy the days patients had been on mechanical ventilation, as well as mortality were taken into consideration. To assess safety the absence of clinical complications potentially attributed to the administration of plasma was considered as well. Inclusion criteria were patients over 18 years-old admitted with mild, moderate, or severe ARDS or according to Berlin Definition and who required mechanical ventilation. The presence of SARS-CoV-2 for the diagnosis of COVID-19 was confirmed after the detection of viral genome using multiple quantitative RT-PCR testing. Nucleic acids were purified using the MagNa Pure 96 System (Roche, Geneva, Switzerland). Extractions underwent an amplification reaction using the TaqMan Fast 1-Step Master Mix (Life technologies, Carlsbad, CA, United States) plus a mix of primers (Thermo Fisher Scientific, Walthman, MA, United States) and taqman MGB probes (Applied Biosystems, Foster City, CA, United States) directed against 2 different targets: ORF1ab and N. Amplifications and further analyses were conducted using the Applied Biosystems 7500 Real-time PCR System.
Individuals from the control group were patients matched by age, sex, and severity admitted to the ICU during the same study period who met some exclusion criterion (presence of antibodies in plasma and date of ICU admission after the first 10 days since symptom onset). Statistical analysis: qualitative variables were expressed as absolute frequencies and percentages. Quantitative variables were expressed as mean, median, and standard deviation Comparative analysis was conducted using the Student-Welch, the chi-square tests, and the Kaplan-Meier survival analysis calculator. All P values and confidence intervals were estimated and assessed using a bilateral 95% confidence level. The study was approved by the research ethics committee of Principality of Asturias, Spain (code #2020.196).
Plasma from convalescent patients was obtained through altruistic donation from patients who had recovered from COVID-19. Only convalescent patients with anti-SARS-CoV-2 antibody values 5 times higher than the cut-off value considered positive were considered eligible for donation. Antibody count was conducted using the fully automated chemiluminescence analyzer LIAISONXL® (LIAISON® SARS-CoV-2 TrimericS IgG, DiaSorin, VC, Italy). Results are presented in arbitrary units per mL (AU/mL) with a lower limit of 13 AU/mL, and a maximum response of 800 AU/mL. Plasma was obtained through plasmapheresis at the Community Blood Center Regional Tissue Bank. A total of 500–600 mL of plasma were drawn in each donation. Afterwards, a process of viral inactivation with methylene blue dye followed that was frozen until used according to standard procedures in place at the blood bank. Patients were infused with 2 bags of PCP for a total of 500 mL.
The study was conducted on a total of 81 patients, 28 of whom were treated with PCP and 53 were considered controls. Patients were matched according to age, sex, and APACHE II score at admission. No significant differences were reported between both groups (Table 1). A total of 35% of the patients were women, and the mean age was 67.6 years. The overall mortality rate was 38.2%. Patients were treated with PCP for a median of 9 days since symptom onset and 2 days since ICU admission. From symptom onset until intubation, the mean of patients who received convalescent plasma was 8.3 days (3–17) compared to 9.1 days (1–16) from the control group.
Clinical characteristics and results from the 2 groups of patients.
| Patients on PCPN = 28 | Patients without PCPN = 53 | P | |
|---|---|---|---|
| Women [n (%)] | 10 (35.7) | 18 (34) | .87 |
| Age [mean (SD)] | 67 (13.4) | 67.4 (11.4) | .87 |
| APACHE II score at admission [mean (SD)] | 16.4 (4.9) | 16.1 (4.4) | .79 |
| Days on MV [median] | 10 | 15 | .18 |
| Days at the ICU [median] | 10 | 16 | .66 |
| Days at the hospital [median] | 26 | 32 | .50 |
| Dead [n (%] | 13 (46.4) | 18 (33.9) | .27 |
MV, mechanical ventilation; PCP, plasma from convalescent patients; SD, standard deviation.
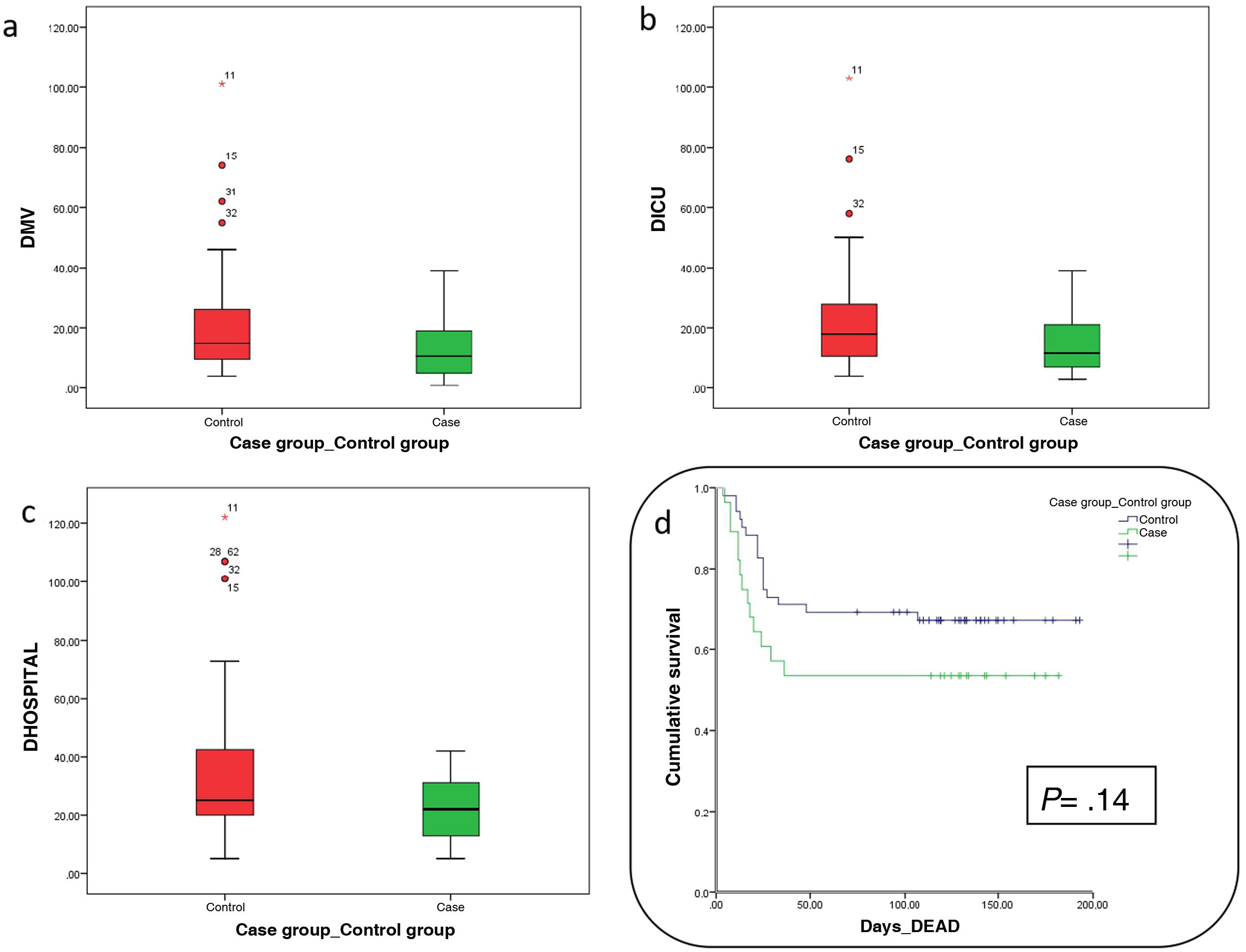
Days at the ICU or at the hospital, and days on mechanical ventilation were fewer in the group of patients treated with PCP although a statistically significant difference could not be seen (Table 1 and Fig. 1). Comparing these same variables among survivors from each group, patients treated with PCP had been a median of 8 days on mechanical ventilation compared to the 14 days of patients naïve to treatment (P = .19), ICU stays of 10 days compared to 16 days in patients naïve to treatment (P = .66), and hospital stays of 26 days compared to 32 days (P = .50).
The mortality rate of patients treated with PCP was 46.4% compared to 33.9% of patients from the control group (P = .27). Analyzing the Kaplan-Meier survival chart, P values = .14 were obtained (Table 1 and Fig. 1). No adverse events associated with the use of PCP were reported.
Therefore, in our own experience, the transfusion of plasma from convalescent patients to critically ill patients with SARS-CoV-2 induced pneumonia does not reduce mortality, the days on mechanical ventilation or the ICU stay. Some study limitations are its small sample size that lacks the necessary statistical power to be able to draw definitive conclusions, and the method used to select both cases and control patients.
Despite the fact that other infectious diseases have had good results with the use of PCP our findings are consistent with those obtained from different studies conducted during the past 2 pandemic years. These findings tell us that no clinical benefits have been reported in severe patients8, or in patients with mild symptoms as possible inhibitor of disease progression into severe stages of the disease9. However, a study recently published found a lower risk of disease progression into severe forms of the disease in patients treated with PCP within the first 9 days since symptom onset10. Currently, although it is not recommended to administer PCP systematically in severe cases of COVID-19, its efficacy in mild patients or other subgroup of immunosuppressed patients remains uncertain.
Authors’ contributionsIván Astola and Dolores Escudero drafted the manuscript. Iván Astola, María Martínez, Ángeles Fernández, and Eva Martínez were involved in data curation. Ana María Ojea, and Pablo Herrero selected the donors. All the authors reviewed the manuscript.
FundingThis research study was funded by the Fundación para la Investigación Biosanitaria de Asturias(FIMBA).
Research Ethics Committee Approval with code #2020.196.
Conflicts of interestNone whatsoever.
We wish to thank all COVID-19 convalescent patients who donated their plasma for their generosity that made this study possible. Our gratitude also goes to the Spanish Society of Medicine and Plastic Surgery for the donation made on behalf of Fundación para la Investigación Biosanitaria de Asturias (FIMBA) to be able to conduct this study.