To describe and compare the demographic characteristics and comorbidities of patients with COVID-19 who died in Spanish hospitals during the 2020 pandemic based on whether they were or were not admitted to an intensive care unit (ICU) prior to death.
MethodsWe performed a secondary analysis of COVID-19 patients who died during hospitalization included by 62 Spanish emergency departments in the SIESTA cohort. We collected the demographic characteristics and comorbidities, determined both individually and estimated globally by the Charlson index (ChI). Independent factors related to ICU admission were identified and different analyses of sensitivity were performed to contrast the consistency of the findings of the principal analysis.
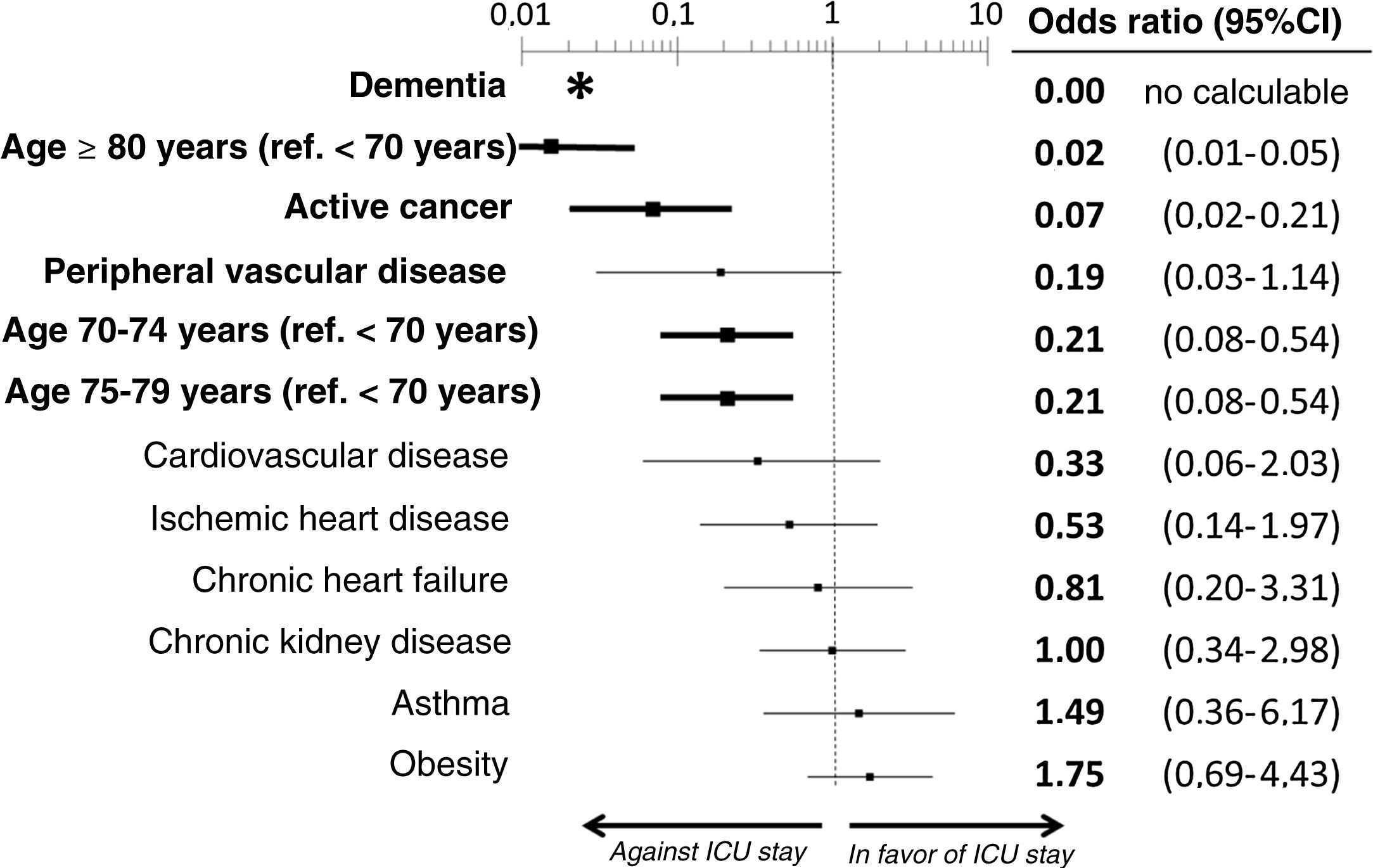
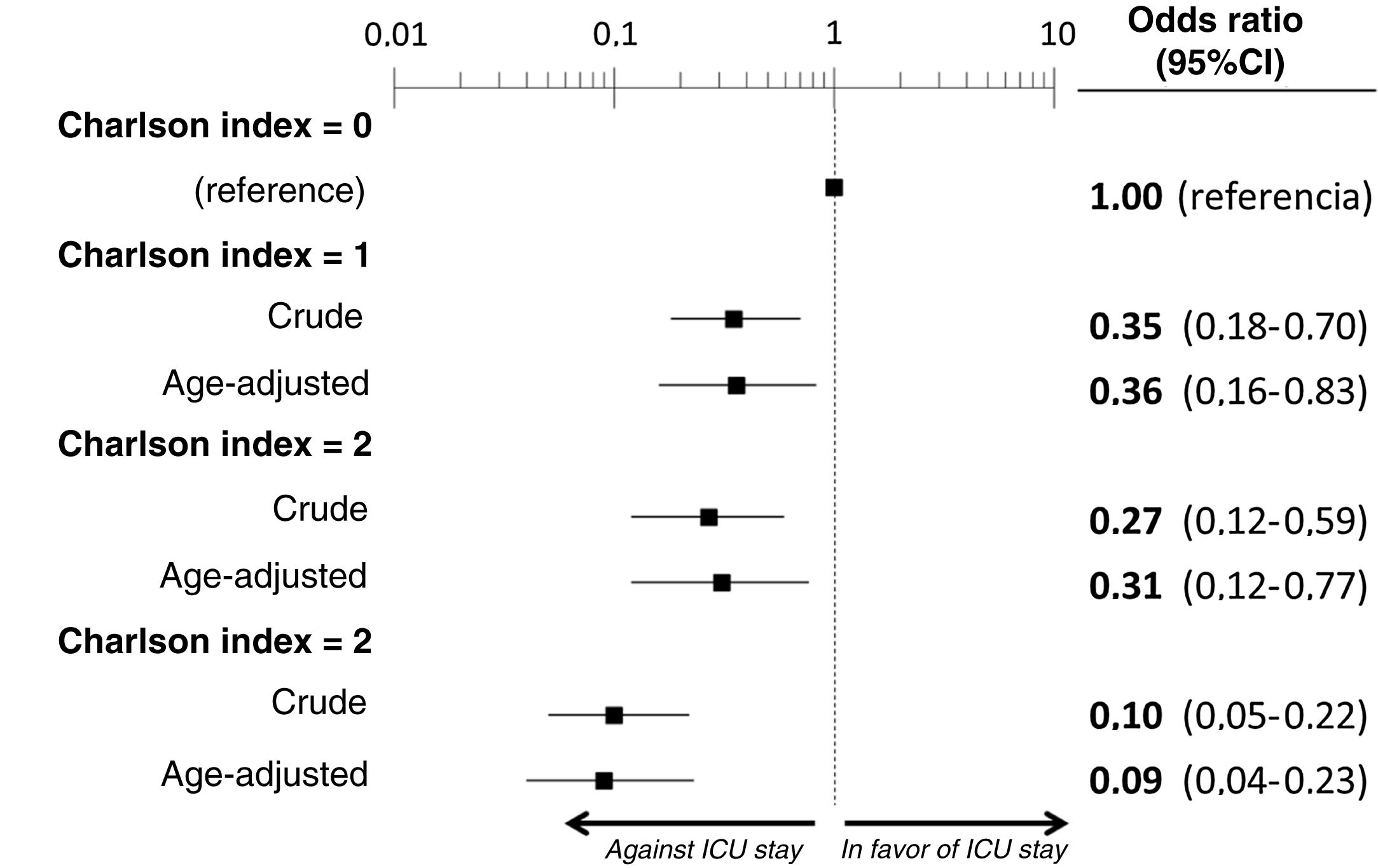
ResultsWe included the 338 patients from the SIESTA cohort that died during hospitalization. Of these, 77 (22.8%) were admitted to an ICU before dying. After multivariate adjustment, 3 out of the 20 basal characteristics analyzed in the present study were independently associated with ICU admission: dementia (no patients with dementia who died were admitted to the ICU: OR=0, 95%CI=not calculable), active cancer (OR=0.07; 95%CI=0.02–0.21) and age (<70 years: OR=1, reference; 70–74 years: OR=0.21; 95%CI=0.08–0.54; 75–79 years: OR=0.21; 95%CI=0.08–0.54; ≥80 years: OR=0.02; 95%CI=0.01–0.05). The probability of ICU admission significantly increased in parallel to the ChI, even after adjustment for age (ChI 0 points: OR=0, reference; ChI 1 point: OR=0.36; 95%CI=0.16–0.83; ChI 2 points: OR=0.36; 95%CI=0.16–0.83; ChI >2 points: OR=0.09; 95%CI=0.04–0.23). The sensitivity analyses showed no gross differences compared to the principal analysis.
ConclusionsThe profile of COVID-19 patients who died without ICU admission is similar to that observed in the usual medical practice before the pandemic. The basal characteristics limiting their admission were age and global burden due to comorbidity, especially dementia and active cancer.
Describir las características demográficas y de comorbilidad de los pacientes con COVID-19 fallecidos en hospitales españoles durante el brote pandémico de 2020 y compararlas según si ingresaron o no en una Unidad de Cuidados Intensivos (UCI) antes del fallecimiento.
MétodosAnálisis secundario de los pacientes de la cohorte SIESTA (formada por pacientes COVID de 62 hospitales españoles) fallecidos durante la hospitalización. Se recogieron sus características demográficas y comorbilidades, individuales y globalmente, estimadas mediante el índice de comorbilidad de Charlson (ICC). Se identificaron los factores independientes relacionados con ingreso en UCI, y se realizaron diversos análisis de sensibilidad para contrastar la consistencia de los hallazgos del análisis principal.
ResultadosSe incluyeron los 338 pacientes de la cohorte SIESTA fallecidos; de ellos, 77 (22,8%) accedieron a una UCI previamente al fallecimiento. En el análisis multivariable, tres de las 20 características basales analizadas se asociaron independientemente con ingreso en UCI de los pacientes fallecidos: demencia (no hubo pacientes fallecidos con demencia que ingresasen en UCI; OR = 0, IC 95% = no calculable), cáncer activo (OR = 0,07, IC 95% = 0,02-0,21) y edad (<70 años: OR = 1, referencia; 70-74 años: OR = 0,21, IC 95% = 0,08-0,54; 75-79 años: OR = 0,21, IC 95% = 0,08-0,54; ≥ 80 años: OR = 0,02, IC 95% = 0,01-0,05). La probabilidad de ingreso en UCI de los pacientes que fallecieron disminuyó significativamente al aumentar el ICC, incluso tras ajustarla por edad (ICC 0 puntos: OR = 1, referencia; ICC 1 punto: OR = 0,36, IC 95% = 0,16-0,83; ICC 2 puntos: OR = 0,36, IC 95% = 0,16-0,83; ICC > 2 puntos: OR = 0,09, IC 95% = 0,04-0,23). Los análisis de sensibilidad no mostraron diferencias destacables respecto al análisis principal.
ConclusionesEl perfil de los pacientes COVID fallecidos sin ingresar en UCI se ajustó a lo observado en la práctica médica habitual antes de la pandemia, y las características basales que limitaron su ingreso fueron la edad y la carga de comorbilidad global, especialmente la demencia y el cáncer activo.
The pandemic caused by SARS-CoV-2 infection has resulted in the greatest healthcare crisis seen in recent times. The enormous number of patients with coronavirus disease 2019 (COVID-19) attended by the healthcare systems of those countries hardest hit by the pandemic caused these systems to become overwhelmed from the early stages. This made it necessary to implement organizational and care strategies at all levels: primary care, out- and in-hospital emergency care, conventional hospitalization, admission to the Intensive Care Unit (ICU), and hospitalization in unconventional alternative centers or units of different kinds.1–5 Another fundamental aspect of the healthcare systems that showed important weakening during the pandemic is referred to the provision of adequate equipment, devices and facilities for effective patient management, protection of the healthcare professionals, and prevention of the spread of the infection within the healthcare system itself – these being circumstances already seen in the past in other highly transmissible infectious processes such as influenza.6–8
In this scenario, one of the main problems in the context of an overwhelmed healthcare system was the assignment of ICU beds for those patients that suffered worsening of their clinical condition. Although during the pandemic up to 300% more critical care beds were habilitated in Spain in hospitals,9 this did not prevent the ICUs from also becoming overwhelmed in certain cities or regions (Autonomous Communities) of the country during a number of days in the course of the pandemic. Different societies protocolized a series of principles for the assignment of hospitalization beds in the ICU that were embodied in recommendations characterized by important consensus and in most cases with common positionings.10–12 However, debate has arisen in this scenario in both the population and in political circles regarding potential inadequacies in the decision to admit patients to the ICU13,14 and potential medical responsibilities in the case of patients with COVID-19 who died without having been admitted to the ICU. The present study explores the basal characteristics of a large and geographically diverse sample of COVID-19 patients that died during the peak of the pandemic, establishing comparisons between those with prior admission to the ICU and those who died without having been admitted to the ICU. The main objective was to determine whether application was made of the standard criteria for patient admission to the ICU, which are fundamentally based on patient age and prior comorbidity.15,16
MethodStudy designThe present study comprises a secondary analysis of the COVID-19 patients included in the UMC-19 (Unusual Manifestations of COVID-19) project, carried out in the 62 Spanish Hospital Emergency Departments (HEDs) forming part of the SIESTA (Spanish Investigators in Emergency Situations Team) research network. These 62 HEDs were selected on a discretional basis for having previously collaborated in other research projects with the coordinators of the SIESTA network, and were located in 12 of the 17 Spanish Autonomous Communities. This geographical distribution ensured a balanced representation according to the local impact or repercussion (care burden) of the pandemic during the study period, with 17 HEDs (27%) presenting low repercussion (<5% of the patients attended during the study period were diagnosed with COVID-19), 21 (34%) average repercussion (5–15% of the patients) and 24 (39%) high repercussion (>15% of the patients). Sixty hospitals of the SIESTA network belong to the public healthcare system and two belong to private hospitals that admitted patients under the charge of public healthcare during the pandemic. In relation to the size of the center to which the HEDs belonged, 27 (44%) were large (>500 beds), 25 (40%) were of average size (200–50 beds), and 10 (16%) were small (<200 beds). The rest of the details of the UMC-19 project and SIESTA network can be consulted in other publications.17,18
The patient inclusion period was from 1 March to 30 April 2020. The patients with COVID-19 were defined as those with nasopharyngeal swab samples proving positive for viral RNA based on reverse transcriptase polymerase chain reaction (RT-PCR) testing either in the emergency department or during hospital stay, if so advised by clinical suspicion and the protocols in force in each moment. However, due to the limited availability of reagents during the peak of the pandemic in Spain and the possibility of false-negative readings with the PCR test, patients with a clinical diagnosis in the emergency department, in the absence of microbiological confirmation, were also accepted.
The present study analyzes all the patients with COVID-19 included in the UMC-19 project that died during hospital stay. These patients with COVID-19 corresponded to two different profiles: patients attended on the basis of their general clinical and typical respiratory manifestations directly derived from the viral infection itself (group A), and patients who in addition to such general manifestations were also diagnosed in the emergency department with some manifestation or complication potentially associated to SARS-CoV-2 infection (deep venous thrombosis, pulmonary embolism, acute coronary syndrome, stroke, upper gastrointestinal bleeding, spontaneous pneumothorax, pericarditis, myopericarditis, meningitis, encephalitis, Guillain-Barré syndrome, pancreatitis) (group B). The latter group of patients included all those individuals with COVID-19 diagnosed with some of these conditions in the 62 participating HEDs during the two months of the study, while group A included randomly selected patients. Randomization was carried out by inclusion of the patient diagnosed with COVID-19 in these HEDs immediately before or after each patient pertaining to group B (or both, for some of the manifestations, if the contemplated number of cases was low).
Study variablesFor all the deceased patients of the SIESTA cohort included in the study, we recorded the following baseline variables: age, gender and 18 comorbidities (arterial hypertension, dyslipidemia, diabetes mellitus, active smoking, obesity [assessed clinically], coronary disease, chronic heart failure, peripheral vascular disease, cerebrovascular disease, chronic kidney disease [creatinine >2mg/dl], chronic obstructive pulmonary disease [COPD], asthma, active cancer, dementia, chronic liver disease, connective tissue disease, AIDS, gastroduodenal ulcer). In addition, calculation was made of the Charlson comorbidity index,19 which based on a single score rates the comorbidity burden resulting from the existence and severity of 13 disease conditions prior to development of the acute process. Likewise, we recorded the time elapsed from the onset of symptoms of COVID-19 to admission to the emergency department, and the complementary tests and treatments started in emergency care. In contrast, management during the hospital admission phase and until the death of the patient was not recorded.
The variable classifying the deceased patients analyzed in the present study was admission to the ICU, either directly after being seen in the emergency department or coming from a conventional hospital ward after a number of days of stay. In addition, we recorded whether the patient underwent orotracheal intubation (OTI) and invasive mechanical ventilation (IMV) in the ICU prior to death.
All the data were obtained through retrospective review of the emergency care and hospitalization case histories. This process was done in each center by the investigators associated to the project, using a customized datasheet. Final assignment of the diagnosis of COVID-19 and of the ICU admission classification variable, prior to death, was made locally, without external monitoring.
Ethical considerationsThe UMC-19 project was approved by the Clinical Research Ethics Committee of Hospital Clínic de Barcelona (Spain) (protocol HCB/2020/0534). In view of the need for urgent data compilation, the obtainment of written informed consent was not considered necessary. The data were analyzed and interpreted by the authors. The database was accessed under conditions of adequate patient coding, in order to ensure anonymity. In no case did we analyze the particular actions of the physicians attending a given patient, and all the considerations in the article are made from the perspective of the global patient sample.
Statistical analysisThe descriptive analysis was based on the calculation of absolute values and percentages in the case of qualitative variables, and the median and interquartile range (IQR) in the case of continuous variables. The two groups of deceased patients, with and without admission to the ICU, were compared using the χ2 test (or Fisher exact test, as applicable) and the Mann–Whitney U-test for qualitative or quantitative variables, respectively. The estimation of the differences between groups was expressed as the odds ratio (OR), with the corresponding 95% confidence interval (95%CI). Continuous variables were grouped using cut-off values of clinical significance where possible, or establishing approximations to medians and quartiles, as applicable in each case. Independent associations were explored by calculating the adjusted odds ratio (aOR) through logistic regression analysis. In this regard, those basal characteristics of the deceased patients that were found in the univariate analysis to exhibit a significantly different distribution between the two groups were entered in the multivariate model. As sensitivity analysis, we repeated this adjustment model in the main analysis but independently for group A (patients with common manifestations of COVID-19; sensitivity analysis S1) and for group B (patients who in addition to the common manifestations of the disease were diagnosed in emergency care with manifestations or complications not common in COVID-19; sensitivity analysis S2), and reclassifying the patients of the group that died in the ICU who did not receive OTI/IMV as patients of the NO-ICU group (on the grounds that some therapeutic ceiling may have been applied; sensitivity analysis S3). Lastly, a segmented analysis was made of the association of the variables found to be independently associated to death with ICU stay, according to whether the HED presented high COVID-19 care burden (>15% of cases of COVID-19 among the total cases attended in emergency care) or not, and we explored whether there was an interaction between such variables and COVID-19 care burden. Differences between groups were considered statistically significant for p<0.05 or if the 95%CI of the OR excluded the value 1. The SPSS version 26 statistical package (IBM, Armonk, NY, USA) was used throughout.
ResultsCharacteristics of the deceased patientsThe study included 338 patients with COVID-19 that died during admission (142 corresponding to group A and 196 to group B). Confirmation of COVID-19 was established by RT-PCR in 299 cases (88.5%; 94.4% in group A and 84.2% in group B; p=0.004). The median time from COVID-19 symptoms onset to admission to the emergency department was four days (IQR=1–7), and was longer in the patients of group A (median 5, IQR=2–7) than in those belonging to group B (median 4, IQR=1–7; p=0.048).
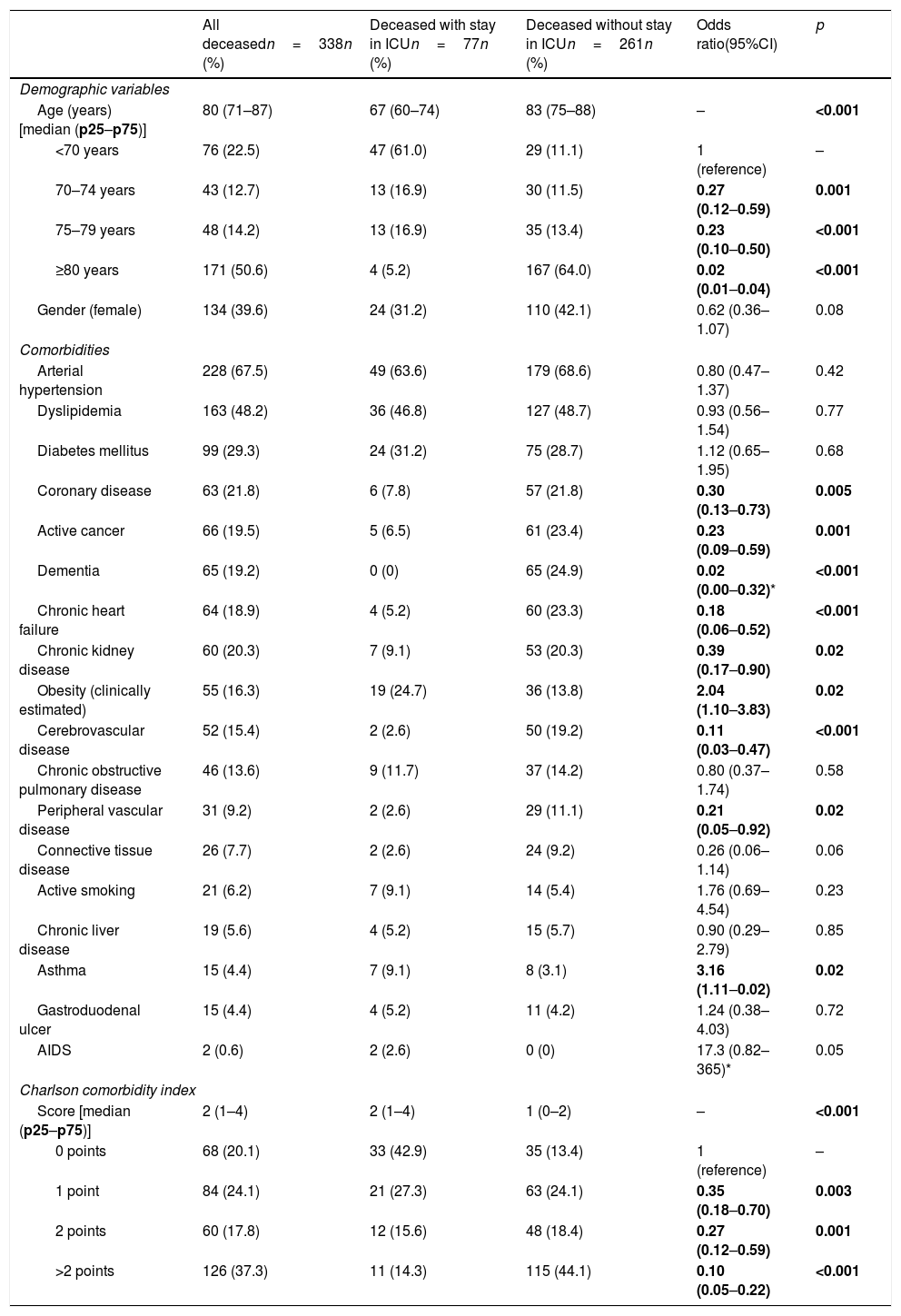
The basal characteristics of the deceased patients are reported in Table 1. The median age was 80 years (IQR=71–87), and 39.6% were women. The most frequent comorbidities were arterial hypertension (67.5%), dyslipidemia (48.2%), diabetes mellitus (29.3%) and coronary disease (21.8%). The median Charlson comorbidity score was 2 (IQR=1–4).
Demographic characteristics, individual comorbidities, and comorbidity index of the patients with COVID-19 that died, with comparison between those admitted to the ICU versus those not admitted to the ICU prior to death.
| All deceasedn=338n (%) | Deceased with stay in ICUn=77n (%) | Deceased without stay in ICUn=261n (%) | Odds ratio(95%CI) | p | |
|---|---|---|---|---|---|
| Demographic variables | |||||
| Age (years) [median (p25–p75)] | 80 (71–87) | 67 (60–74) | 83 (75–88) | – | <0.001 |
| <70 years | 76 (22.5) | 47 (61.0) | 29 (11.1) | 1 (reference) | – |
| 70–74 years | 43 (12.7) | 13 (16.9) | 30 (11.5) | 0.27 (0.12–0.59) | 0.001 |
| 75–79 years | 48 (14.2) | 13 (16.9) | 35 (13.4) | 0.23 (0.10–0.50) | <0.001 |
| ≥80 years | 171 (50.6) | 4 (5.2) | 167 (64.0) | 0.02 (0.01–0.04) | <0.001 |
| Gender (female) | 134 (39.6) | 24 (31.2) | 110 (42.1) | 0.62 (0.36–1.07) | 0.08 |
| Comorbidities | |||||
| Arterial hypertension | 228 (67.5) | 49 (63.6) | 179 (68.6) | 0.80 (0.47–1.37) | 0.42 |
| Dyslipidemia | 163 (48.2) | 36 (46.8) | 127 (48.7) | 0.93 (0.56–1.54) | 0.77 |
| Diabetes mellitus | 99 (29.3) | 24 (31.2) | 75 (28.7) | 1.12 (0.65–1.95) | 0.68 |
| Coronary disease | 63 (21.8) | 6 (7.8) | 57 (21.8) | 0.30 (0.13–0.73) | 0.005 |
| Active cancer | 66 (19.5) | 5 (6.5) | 61 (23.4) | 0.23 (0.09–0.59) | 0.001 |
| Dementia | 65 (19.2) | 0 (0) | 65 (24.9) | 0.02 (0.00–0.32)* | <0.001 |
| Chronic heart failure | 64 (18.9) | 4 (5.2) | 60 (23.3) | 0.18 (0.06–0.52) | <0.001 |
| Chronic kidney disease | 60 (20.3) | 7 (9.1) | 53 (20.3) | 0.39 (0.17–0.90) | 0.02 |
| Obesity (clinically estimated) | 55 (16.3) | 19 (24.7) | 36 (13.8) | 2.04 (1.10–3.83) | 0.02 |
| Cerebrovascular disease | 52 (15.4) | 2 (2.6) | 50 (19.2) | 0.11 (0.03–0.47) | <0.001 |
| Chronic obstructive pulmonary disease | 46 (13.6) | 9 (11.7) | 37 (14.2) | 0.80 (0.37–1.74) | 0.58 |
| Peripheral vascular disease | 31 (9.2) | 2 (2.6) | 29 (11.1) | 0.21 (0.05–0.92) | 0.02 |
| Connective tissue disease | 26 (7.7) | 2 (2.6) | 24 (9.2) | 0.26 (0.06–1.14) | 0.06 |
| Active smoking | 21 (6.2) | 7 (9.1) | 14 (5.4) | 1.76 (0.69–4.54) | 0.23 |
| Chronic liver disease | 19 (5.6) | 4 (5.2) | 15 (5.7) | 0.90 (0.29–2.79) | 0.85 |
| Asthma | 15 (4.4) | 7 (9.1) | 8 (3.1) | 3.16 (1.11–0.02) | 0.02 |
| Gastroduodenal ulcer | 15 (4.4) | 4 (5.2) | 11 (4.2) | 1.24 (0.38–4.03) | 0.72 |
| AIDS | 2 (0.6) | 2 (2.6) | 0 (0) | 17.3 (0.82–365)* | 0.05 |
| Charlson comorbidity index | |||||
| Score [median (p25–p75)] | 2 (1–4) | 2 (1–4) | 1 (0–2) | – | <0.001 |
| 0 points | 68 (20.1) | 33 (42.9) | 35 (13.4) | 1 (reference) | – |
| 1 point | 84 (24.1) | 21 (27.3) | 63 (24.1) | 0.35 (0.18–0.70) | 0.003 |
| 2 points | 60 (17.8) | 12 (15.6) | 48 (18.4) | 0.27 (0.12–0.59) | 0.001 |
| >2 points | 126 (37.3) | 11 (14.3) | 115 (44.1) | 0.10 (0.05–0.22) | <0.001 |
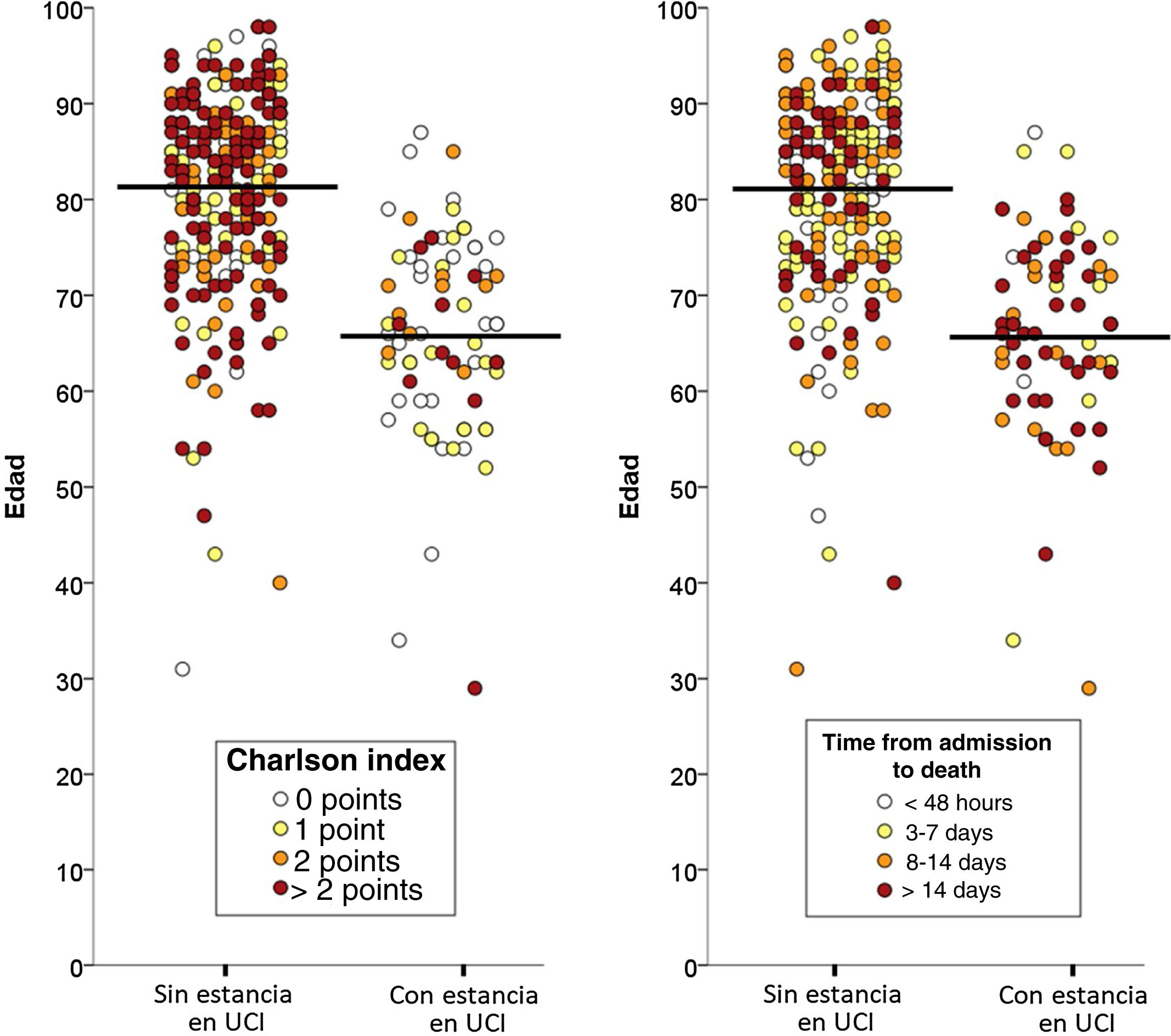
Of the total 388 patients that died during admission, 77 (22.8%) were admitted to the ICU and 261 (77.2%) died without having been admitted to the ICU. The median time from hospital admission to death was 8 days (IQR=3–16), and proved longer among the patients that died admitted to the ICU (median 8, IQR=2–16) than in those that died without admission to the ICU (median 3, IQR=1–7; p<0.001). The two groups of patients differed significantly in 10 of the 20 characteristics analyzed: age, coronary disease, chronic kidney disease, active cancer, dementia, chronic heart failure, cerebrovascular disease and chronic obstructive pulmonary disease were more prevalent among the deceased patients that were not admitted to the ICU, while obesity, asthma and AIDS were more prevalent among those that died with admission to the ICU (Table 1). The median Charlson comorbidity score was 1 (IQR=0–2) in the patients admitted to the ICU and 2 (IQR=1–4) among those not admitted to the ICU (p<0.001). The relationships between age and the Charlson index, and between age and the time elapsed from admission to death of the patients, with and without admission to the ICU, are reported in Fig. 1.
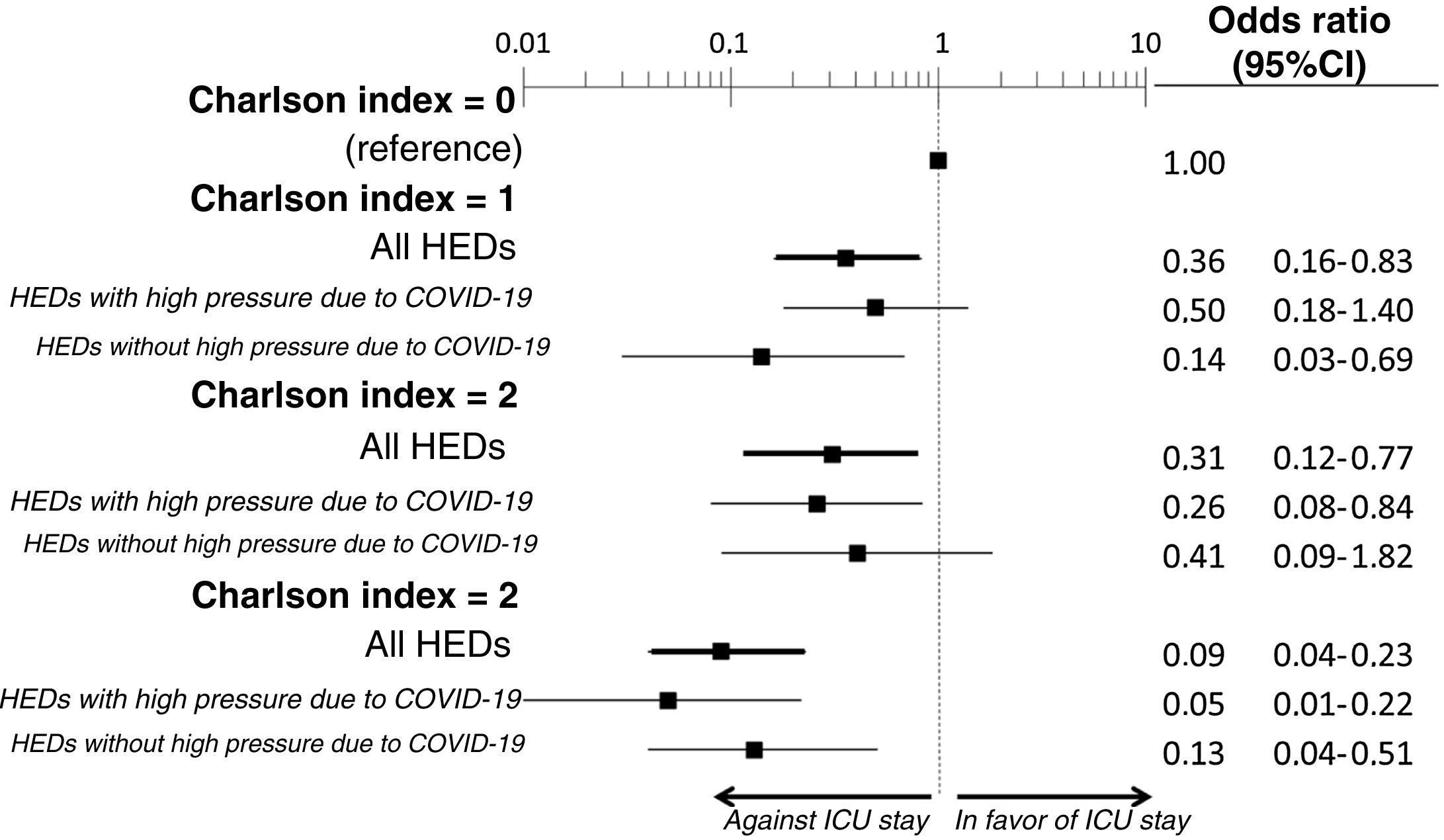
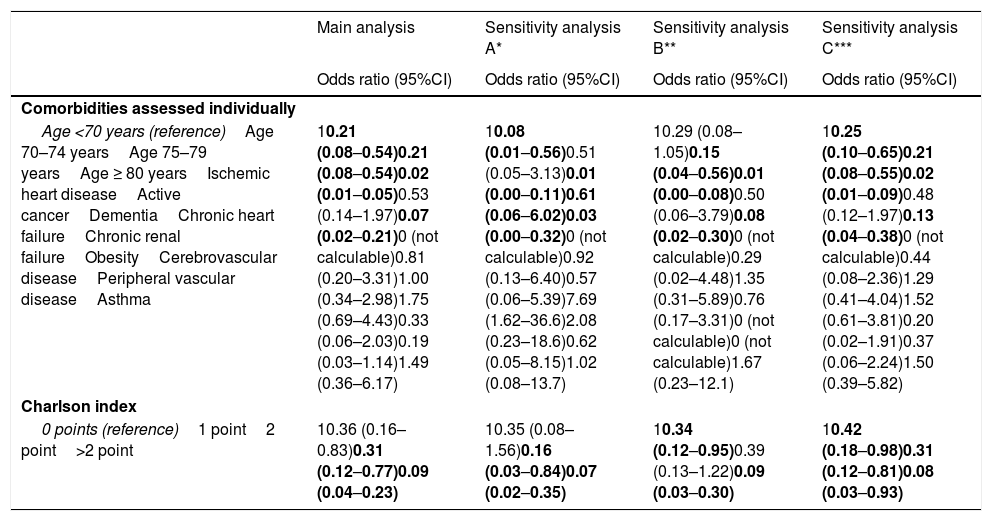
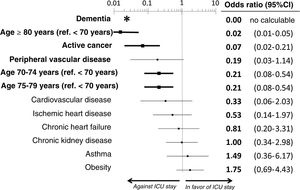
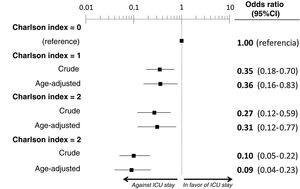
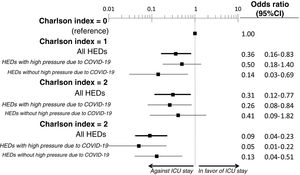
The multivariate analysis comprising the 10 variables individually found to be significant in the univariate analysis showed only three of them to be independently correlated to death with prior admission to the ICU: dementia (OR=0, 95%CI not calculable, since there were no deceased patients with dementia admitted to the ICU), active cancer (OR=0.07, 95%CI=0.02–0.21) and age (in relation to the patients <70 years of age, those aged 70–74 presented an OR of death with prior admission to the ICU of 0.21, 95%CI=0.08–0.54; those aged 75–79 years presented an OR of 0.21, 95%CI=0.08–0.54; and those aged ≥80 years presented an OR of 0.02, 95%CI=0.01–0.05) (Fig. 2). Likewise, we observed a statistically significant decrease in the probability of death with prior admission to the ICU as the Charlson comorbidity score increased – both when analyzed on a crude basis and after adjusting for patient age (Fig. 3). The segmented analysis of the 198 patients from hospitals with a high care burden and the 140 patients from hospitals without a high care burden revealed no significant differences (interaction p-value: 0.33) regarding the type of hospital and the impact of comorbidity burden (Charlson score), adjusted for age, regarding the probability of death with prior admission to the ICU (Fig. 4).
Estimation of the effect of the different basal characteristics of the deceased patients with COVID-19 included in the multivariate model upon the probability of admission to the ICU before death.
*No patients with dementia were admitted to the ICU; the confidence interval therefore cannot be calculated.
Ref.: reference; CI: confidence interval; ICU: Intensive Care Unit.
The variables in boldface showed statistically significant differences (p<0.05).
Segmented analysis according to care burden in the emergency department during the pandemic of the age-adjusted effect of comorbidity burden (Charlson index) upon the probability of admission of the patients with COVID-19 to the ICU before death (interaction p-value: 0.33).
ICU: Intensive Care Unit; HED: Hospital Emergency Department.
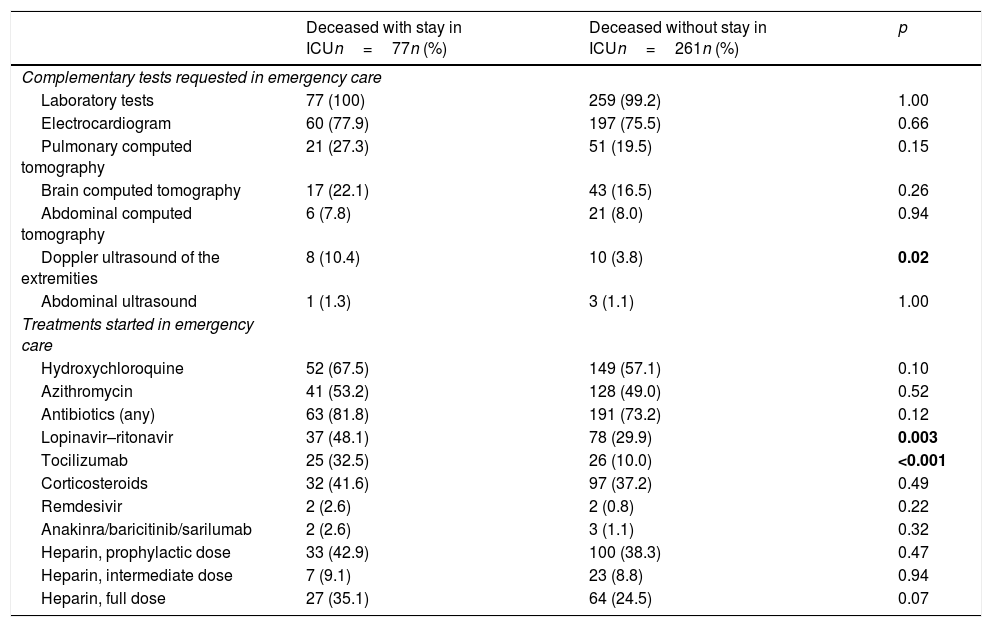
On the other hand, in relation to care in the emergency department, the differences between the two groups were few (Table 2), and only an increased use of Doppler ultrasound and of the prescription of ritonavir–lopinavir and tocilizumab was observed among those patients that died with admission to the ICU.
Treatments and tests during stay in the emergency department in the patients with COVID-19 that died with and without prior admission to the ICU.
| Deceased with stay in ICUn=77n (%) | Deceased without stay in ICUn=261n (%) | p | |
|---|---|---|---|
| Complementary tests requested in emergency care | |||
| Laboratory tests | 77 (100) | 259 (99.2) | 1.00 |
| Electrocardiogram | 60 (77.9) | 197 (75.5) | 0.66 |
| Pulmonary computed tomography | 21 (27.3) | 51 (19.5) | 0.15 |
| Brain computed tomography | 17 (22.1) | 43 (16.5) | 0.26 |
| Abdominal computed tomography | 6 (7.8) | 21 (8.0) | 0.94 |
| Doppler ultrasound of the extremities | 8 (10.4) | 10 (3.8) | 0.02 |
| Abdominal ultrasound | 1 (1.3) | 3 (1.1) | 1.00 |
| Treatments started in emergency care | |||
| Hydroxychloroquine | 52 (67.5) | 149 (57.1) | 0.10 |
| Azithromycin | 41 (53.2) | 128 (49.0) | 0.52 |
| Antibiotics (any) | 63 (81.8) | 191 (73.2) | 0.12 |
| Lopinavir–ritonavir | 37 (48.1) | 78 (29.9) | 0.003 |
| Tocilizumab | 25 (32.5) | 26 (10.0) | <0.001 |
| Corticosteroids | 32 (41.6) | 97 (37.2) | 0.49 |
| Remdesivir | 2 (2.6) | 2 (0.8) | 0.22 |
| Anakinra/baricitinib/sarilumab | 2 (2.6) | 3 (1.1) | 0.32 |
| Heparin, prophylactic dose | 33 (42.9) | 100 (38.3) | 0.47 |
| Heparin, intermediate dose | 7 (9.1) | 23 (8.8) | 0.94 |
| Heparin, full dose | 27 (35.1) | 64 (24.5) | 0.07 |
ICU: Intensive Care Unit.
The figures in boldface correspond to comparisons with statistically significant differences (p<0.05).
The individualized analysis of groups A and B of deceased patients with COVID-19 (sensitivity analysis A and B, respectively) again identified as independent factors of admission to the ICU the same factors as those identified in the main analysis (age, dementia and active cancer, as well as comorbidity burden considered globally on the basis of the Charlson score). In addition, in group A, obesity was associated to an increased probability of death with prior admission to the ICU, and in group B (with complications added to the clinical manifestations of COVID-19) there were no patients with cerebrovascular disease or peripheral vascular disease that died having been admitted to the ICU; their OR of admission to the ICU was therefore 0 (95%CI not calculable in either case) (Table 3).
Results of the sensitivity analysis of the adjusted estimations of the effect of the basal characteristics of the patients with COVID-19 that died upon admission to the ICU before death.
| Main analysis | Sensitivity analysis A* | Sensitivity analysis B** | Sensitivity analysis C*** | |
|---|---|---|---|---|
| Odds ratio (95%CI) | Odds ratio (95%CI) | Odds ratio (95%CI) | Odds ratio (95%CI) | |
| Comorbidities assessed individually | ||||
| Age <70 years (reference)Age 70–74 yearsAge 75–79 yearsAge ≥ 80 yearsIschemic heart diseaseActive cancerDementiaChronic heart failureChronic renal failureObesityCerebrovascular diseasePeripheral vascular diseaseAsthma | 10.21 (0.08–0.54)0.21 (0.08–0.54)0.02 (0.01–0.05)0.53 (0.14–1.97)0.07 (0.02–0.21)0 (not calculable)0.81 (0.20–3.31)1.00 (0.34–2.98)1.75 (0.69–4.43)0.33 (0.06–2.03)0.19 (0.03–1.14)1.49 (0.36–6.17) | 10.08 (0.01–0.56)0.51 (0.05–3.13)0.01 (0.00–0.11)0.61 (0.06–6.02)0.03 (0.00–0.32)0 (not calculable)0.92 (0.13–6.40)0.57 (0.06–5.39)7.69 (1.62–36.6)2.08 (0.23–18.6)0.62 (0.05–8.15)1.02 (0.08–13.7) | 10.29 (0.08–1.05)0.15 (0.04–0.56)0.01 (0.00–0.08)0.50 (0.06–3.79)0.08 (0.02–0.30)0 (not calculable)0.29 (0.02–4.48)1.35 (0.31–5.89)0.76 (0.17–3.31)0 (not calculable)0 (not calculable)1.67 (0.23–12.1) | 10.25 (0.10–0.65)0.21 (0.08–0.55)0.02 (0.01–0.09)0.48 (0.12–1.97)0.13 (0.04–0.38)0 (not calculable)0.44 (0.08–2.36)1.29 (0.41–4.04)1.52 (0.61–3.81)0.20 (0.02–1.91)0.37 (0.06–2.24)1.50 (0.39–5.82) |
| Charlson index | ||||
| 0 points (reference)1 point2 point>2 point | 10.36 (0.16–0.83)0.31 (0.12–0.77)0.09 (0.04–0.23) | 10.35 (0.08–1.56)0.16 (0.03–0.84)0.07 (0.02–0.35) | 10.34 (0.12–0.95)0.39 (0.13–1.22)0.09 (0.03–0.30) | 10.42 (0.18–0.98)0.31 (0.12–0.81)0.08 (0.03–0.93) |
This sensitivity analysis only includes those patients with COVID-19 presenting typical general and respiratory manifestations directly attributable to the viral infection (group A, n=142; see Material and Methods).
This sensitivity analysis only includes those patients with COVID-19 who in addition to the general clinical manifestations were also diagnosed in emergency care with some manifestation or complication potentially associated to SARS-CoV-2 infection (deep venous thrombosis, pulmonary embolism, acute coronary syndrome, stroke, upper gastrointestinal bleeding, spontaneous pneumothorax, pericarditis, myopericarditis, meningitis, encephalitis, Guillain-Barré syndrome, pancreatitis; group B; n=195; see Material and Methods).
This sensitivity analysis reclassifies 15 patients admitted to the ICU, but who were not intubated or ventilated in the group of subjects not admitted to the ICU, understanding that some therapeutic limitation had been applied (thus leaving a total of 62 and 276 patients in the groups with and without admission to the ICU, respectively; see Material and Methods).
The figures in boldface correspond to comparisons with statistically significant differences (p<0.05).
On the other hand, 15 of the 77 patients admitted to the ICU (19.5%) died without having undergone OTI/IMV. When these patients changed group (from deceased with admission to the ICU to deceased without admission to the ICU, in the understanding that a therapeutic ceiling or limitation had been applied, sensitivity analysis C), the independent variables associated to death with admission to the ICU and with OTI/IMV were again the same as in the main analysis (dementia, active cancer and age, and global comorbidity burden) (Table 3).
DiscussionThe management of serious acute disease when the patient suffers gradual organ and system failure, using the most aggressive therapeutic measures available in medical practice, is carried out by specialists in intensive care medicine in the setting of the ICU. However, such therapies are not generally applicable to all patients or in all contexts. In this regard, a series of principles must be universally applied with the aim of ensuring that these treatments are adequately adjusted to each individualized patient – since their indiscriminate application can prove futile and is often associated to therapeutic excess. Adequate application is largely fundamented upon due consideration of the age and comorbidities of the patient – these being essential aspects, since they have a direct impact upon the clinical severity of the acute process causing decompensation, the concomitant involvement of other organs, and the potential reversibility of the clinical condition. The present study shows that, in the same way as in routine clinical practice,15,16 the age and global comorbidity burden of the patient (particularly dementia and active cancer) are independent factors associated to death without admission to the ICU. In fact, only 11% of those who died without admission to the ICU were under 70 years of age, and of these, the great majority had important comorbidity. Furthermore, 80.9% of the patients that died without admission to the ICU were hospitalized for at least 48h (95.1% for at least 24h) – this being long enough for consensus-based decision making among the patients, relatives, attending physicians and specialists in intensive care. Thus, it should be considered that in the great majority of cases there was sufficient time for a pondered and consensus-based decision.
With regard to age, it is clear that aging of the population has significant ethical implications for the management of elderly individuals in the ICU. In our study, most of the elderly patients in turn had important morbidity, and most died without having been admitted to the ICU. In effect, only 5% of the deceased patients aged 80 years or older in our series were admitted to the ICU. This is largely in line with the situation found in the ICUs of Lombardy (Italy), where only 1% of 1591 patients admitted to the ICU were over 80 years old, and less than half of them survived.20 However, age considered on an isolated basis should not be used in the assignment of resources in either the general population or in the population with COVID-19.21
In this sense, it is well known that comorbidity exerts a significant and negative influence upon different outcomes of hospital care, such as the duration of stay, complications, disability, chances for rehabilitation, resource utilization and the possibility of surviving the disorder leading to admission in the first place, and moreover results in worsened functional capacity and quality of life after discharge.22 Thus, comorbidity is the other key aspect in addition to age that must be considered in deciding the intensity of management in critical patients. We found dementia and active cancer to be the two comorbidities associated to non-admission to the ICU. Other conditions such as ischemic heart disease, cardiovascular disease and peripheral vascular disease could also be related, though in our study they failed to reach statistical significance. In any case, global comorbidity burden was clearly associated to a lesser probability of admission to intensive care. This comorbidity burden has been consistently more manifest in patients with COVID-19 that died in the larger series from other countries.23,24 Similarly, an analysis of 104 deceased patients in Wuhan (China) showed that those who died without admission to the ICU had greater comorbidity.25
On analyzing the results of our study, it cannot be ruled out that the decisions made in the Spanish ICUs may have been influenced by the poor outcomes reported in previous series (China, Italy) in patients with COVID-19 within a certain age range, or by the recommendations linked to age ranges made by the implicated scientific societies.10–12 All this could have led to exacerbated and premature pessimism, with age being focalized as the predominant factor, and with less consideration of other variables – something that does not happen in other serious diseases (sepsis or pneumonia, to mention just two examples). The fact that the deceased patients in our series had a high median age (80 years) and that the median Charlson score was not particularly high (2 points) would speak in favor of this hypothesis: patients without excessive comorbidity in which age possibly had a greater influence than normal in decision making, particularly in centers where demand at the time clearly outweighed the available resources. Although the ultimate cause underlying the decision was not analyzed in our study, Fig. 1 shows that comorbidity in a certain sense modulated the decision. Lastly, mention must be made of the importance of a practical ethical approach to the admission of these patients to the ICU, combining the classical survival probability factors considered in the our study (comorbidity, age, dependency), as a first approach, with social usefulness criteria in the event of an important disproportion between care offer and demand – in line with the recommendations of recent publications.26
The intensity of management of the patients in the emergency department, according to whether they were admitted to the ICU or not, did not differ in any major ways, beyond the greater use of lopinavir–ritonavir and tocilizumab in those patients that were finally admitted to the ICU. It should be noted that all these treatments were prescribed on an off-label basis, reflecting an attempt to always offer what was considered to possibly improve the condition of the patient, based on the data available at the time. This strategy, defined as living systematic review, has been defended by a number of authors for use during the COVID-19 pandemic, particularly in critical patients requiring admission to the ICU.27,28 It is quite possible that the increased use of lopinavir–ritonavir and of tocilizumab reflects the identification – from the emergency department – of those patients with a clinical profile indicating candidate status for more aggressive management. However, beyond the inherent aggressivity of treatment, it is also important to ensure the early identification of those patients likely to worsen and require intensive support measures.29 It is possible, however, that such early identification, which is routinely made in ordinary care situations, was not feasible during the resource-overwhelming period of the COVID-19 pandemic in the months of March and April 2020.
Lastly, our study does not address the potential effect of the patients admitted to the ICU with therapeutic ceiling upon the observed outcomes. Nevertheless, sensitivity analysis C seeks to offer an estimate in this regard (eliminating the patients that died in the ICU without having undergone OTI/IMV), and shows that the results obtained are very similar in all aspects to those obtained in the main analysis. On the other hand, we now know that certain noninvasive therapies, such as normobaric high-flow oxygen therapy, noninvasive mechanical ventilation, and continuous positive airway pressure (CPAP) devices were little used due to concern about the spread of aerosols in the environment, with secondary contamination of the healthcare staff (particularly at the start of the pandemic, and in the absence of adequate and supervised facilities for such therapies). However, recent studies have shown that these therapies are feasible in patients with COVID-19 and can avoid OTI/VMI in up to 30% of the cases.30,31 Although our study did not document whether these modalities were used in the 19.5% of patients that died in the ICU without OTI/IMV, they should be taken into account in new waves of elderly patients that may respond to such treatments, as a measure of support and mainly in the ICU due to the structural limitations of many centers, before resorting to OTI/IMV.
Our study has a number of limitations. Firstly, although a part of the patients of the SIESTA cohort were selected on a randomized basis (group A), another part (group B) corresponded to patients presenting some specific complication upon arrival in the emergency department. Nevertheless, the sensitivity analysis showed the main factors associated to death with admission to the ICU in both groups to be the same. Secondly, the tests made in the emergency department and RT-PCR confirmation of SARS-CoV-2 infection, the decision referred to admission, the place of admission, and the treatment of the patients were all adjusted to the changing protocols issued by the authorities and the infrastructure and availability of each center. Consequently, they were not homogeneous during the entire period. Thirdly, this is a secondary analysis of an existing cohort that included all the deceased patients, without prior sample size calculation. As a result, in some cases there may have been type II error, and some estimations of the associations between the basal characteristics and admission to the ICU might not have reached statistical significance due to the limited sample size involved. A fourth limitation is the fact that our study assessed age, gender, and comorbidity as basal characteristics. In this regard, other aspects such as frailty or functional dependency have been recommended by experts for better decision making.21,32,33 However, in view of the retrospective nature of our study, these variables could not be compiled. The same applies to certain severity indexes such as the APACHE II and SOFA, for which the patient case histories did not specify the scores, and for which not all the variables were available for calculating the scores – particularly in the patients that died without admission to the ICU. Likewise, we did not determine the degree of ICU occupation in each moment and in each of the hospitals (nor did we assess patient transfer from centers lacking an ICU). Consequently, we cannot determine the impact of the real-time availability of ICU beds upon the described outcomes. A fifth limitation of our study is the fact that among the different treatments applied (particularly in the patients not admitted to the ICU), the use of noninvasive ventilatory therapy was not documented – such therapy possibly having been employed in some cases in the emergency department or in conventional wards or areas habilitated for this purpose as therapeutic ceiling. A sixth limitation is that we intentionally avoided individual assessment of any patient in the series. We therefore cannot rule out the possibility that some of the deaths that occurred without admission to the ICU were sudden deaths with no chance for assessing or requesting admission to intensive care in view of the rapid and unexpected worsening of the patient. A seventh limitation is the fact that the complementary tests and treatments recorded were circumscribed to the emergency care setting, without exploring what happened from that time up until death. An eighth limitation was the fact that pressure upon the ICUs was not homogeneous among all the Autonomous Communities, and thus the application of limitations may have been more intense in some Communities than in others, and even in some concrete centers with respect to others. Although the aim of our study was to afford a global estimate without going into details referred to each Autonomous Community or center, we found the impact of comorbidity (adjusted for age) upon the probability of prior admission to the ICU to be similar in HEDs with a high care burden due to COVID-19 and in the rest of HEDs (p=0.33, for the interaction between comorbidity adjusted for age and care burden in the HED). Lastly, part of our conclusion that ICU admission of the patients with COVID-19 was consistent with routine practice in other disease processes was based on the unconfirmed assumption that patient age and the incidence of comorbidities (particularly dementia and cancer, which were those found to be significantly different) among the patients with COVID-19 that died admitted to the ICU were the same as in other disease conditions giving rise to admission to the ICU.
In conclusion, the profile of the patients with COVID-19 that died without admission to the ICU in Spain during the peak of the 2020 pandemic was consistent with that seen in routine practice before the pandemic, and the basal characteristics limiting admission were age and the global comorbidity burden. With regard to the latter, the comorbidities most closely related to non-admission to the ICU were dementia and active cancer. This is consistent with the observations in series of patients presenting other types of diseases, and with the opinion of the medical community in general.15,16,34 Therefore, this study, which included deceased patients in 62 Spanish hospitals, evidences proportionality in the use of the ICU as a management resource in patients with COVID-19 during the peak of the pandemic.
Contribution of the authorsAll the authors of the present manuscript contributed to the conception and design of the study, and to data acquisition, analysis, and interpretation. Dr. Òscar Miró wrote the article, and the rest of the authors conducted a critical review of the intellectual content, with final approval of the submitted manuscript.
Steering committee: Òscar Miró, Sònia Jiménez (Hospital Clínic, Barcelona), Juan González del Castillo, Francisco Javier Martín-Sánchez (Hospital Clínico San Carlos, Madrid), Pere Llorens (Hospital General de Alicante), Guillermo Burillo-Putze (Hospital Universitario de Canarias, Tenerife), Alfonso Martín (Hospital Universitario Severo Ochoa de Leganés, Madrid), Pascual Piñera Salmerón (Hospital General Universitario Reina Sofía, Murcia).
Participating centers: Hospital Universitario Doctor Peset Aleixandre de Valencia: María Luisa López Grima, María Ángeles Juan Gómez. Hospital Universitario y Politécnico La Fe de Valencia: Javier Millán, Leticia Serrano Lázaro. Hospital Universitario General de Alicante: Tamara García, Ana Belén Paya. Hospital Clínico Universitario de Valencia: José Noceda. Hospital Arnau de Vilanova de Valencia: María José Cano Cano, Rosa Sorando Serra. Hospital Francesc de Borja de Gandía, Valencia: María José Fortuny Bayarri, Francisco José Salvador Suárez. Hospital General Universitario de Elche, Alicante: Matilde González Tejera. Hospital Marina Baixa de Villajoyosa de Alicante: Juan Miguel Marín Porrino, María Rosales Maestre. Hospital Virgen de los Lirios, Alcoy Alicante: Napoleón Meléndez, Patricia Borrás Albero. Hospital Universitario Vinalopó de Elche (Alicante): Julio Armas Castro, Esther Ruescas Escolano Hospital Universitario de Torrevieja de Alicante: Alexandra Milán Mestre, Alba Llario Ángel. Hospital Lluís Alcanyís de Xàtiva: Carles Pérez García, Pilar Sánchez Amador. Hospital Universitario de La Ribera de Valencia: José Vicente Brasó Aznar, José Luis Ruiz López. Hospital de la Vega Baja Orihuela de Alicante: María Belén Rayos Belda, María Ángeles Murcia Herrero. Hospital Universitario Sant Joan de Alicante: Elena Díaz Fernández. Hospital General de Requena de Valencia: Verónica de los Santos, Carol Cuenca Valero. Hospital de Llíria de Valencia: Ana Peiró Gómez, Elena Gonzalo Bellver. Hospital de la Santa Creu i Sant Pau (Barcelona): Álvaro Eugenio Labat Ponsa, Mireia González Alemany. Hospital Clínic (Barcelona): Carlos Cardozo. Hospital Universitari de Bellvitge de Hospitalet de Llobregat (Barcelona): Ferrán Llopis-Roca, Antonio Haro-Bosch. Hospital Universitari Germans Trias i Pujol de Badalona (Barcelona): Josep Maria Mòdol Deltell, Samuel Olmos Soto. Hospital de Terrassa (Barcelona): Josep Tost. Hospital del Mar (Barcelona): Alfonso Aguirre Tejedo, Isabel Cirera Lorenzo. Hospital Universitari Joan XXIII (Tarragona): Anna Palau, Ruth Gaya Tur. Hospital Universitari de Girona Dr. Josep Trueta (Girona): María Adroher Muñoz, Ester Soy Ferrer. Hospital Universitari de Vic (Barcelona): Lluís LLauger García. Hospital de Sant Pau i Santa Tecla (Tarragona): Enrique Martín Mojarro, Brigitte Silvana Alarcón Jiménez. Clínica Sagrada Familia (Barcelona): Arturo Huerta. Hospital Clínico San Carlos (Madrid): Marcos Fragiel. Hospital Universitario La Paz (Madrid): Elena Muñoz del Val, Charbel Maroun Eid. Hospital Universitario de la Princesa (Madrid): Carmen del Arco Galán, Guillermo Fernández Jiménez. Hospital Universitario Severo Ochoa de Leganés (Madrid): María Cruz Yagüe, Dolores Corbacho Loarte. Hospital Universitario Rey Juan Carlos (Madrid): Belén Rodríguez Miranda, Alejandra Sánchez Arias. Hospital Universitario del Henares (Madrid): Martín Ruiz Grinspan, Leticia Gómez Sánchez Hospital Universitario de Fuenlabrada (Madrid): María Jesús Domínguez, María Eugenia Barrero Ramos. Hospital Universitario Infanta Cristina de Parla (Madrid): Ruth Calderón Hernaiz, Cristina Güemes de la Iglesia. Hospital Comarcal El Escorial (Madrid): Sara Gayoso Martín, Frida Vallejo Somohano. Clínica Universidad Navarra de Madrid: Raquel Piñero Panadero, María García-Uría. Hospital Universitario de Salamanca: Manuel Ángel Palomero Martín, Cristina Gil Castillo. Complejo Asistencial Universitario de León: Begoña Rodríguez Suárez, Mónica Santos Orus. Hospital Universitario de Burgos: María Pilar López Díez. Hospital Universitario Río Hortega (Valladolid): María del Mar Villacorta Martín, Rosa Ibán Ochoa. Complejo Asistencial de Soria: Fahd Beddar Chaib, Lucía Hernández Martínez. Hospital Universitario Regional de Málaga: Ana Pérez Tornero, Alberto Núñez Chía. Hospital Universitario Juan Ramón Jiménez: Esther Maldonado Pérez, Verónica Rodríguez Martín. Hospital Costa del Sol de Marbella: Elisa Delgado Padial, Ana Belén García Soto. Hospital Valle de los Pedroches de Pozoblanco (Córdoba): Jorge Pedraza García. Hospital Virgen del Rocío de Sevilla: Amparo Fernández de Simón Almela. Complejo Hospitalario Universitario de A Coruña: Ricardo Calvo López. Hospital Universitario Lucus Augusti Lugo: Juan José López Díaz. Complejo Hospitalario Universitario de Vigo. Hospital Álvaro Cunqueiro: María Teresa Maza Vera, Raquel Rodríguez Calveiro. Hospital Universitario General de Albacete: Francisco Javier Lucas-Imbernón, María Ruipérez Moreno. Hospital Virgen de la Luz (Cuenca): Félix González Martínez, Diana Moya Olmeda. Hospital Nuestra Señora del Prado de Talavera de la Reina (Toledo): Ricardo Juárez. Hospital Universitario de Canarias (Tenerife): Marcos Expósito Rodríguez, Patricia Eiroa Hernández. Hospital Universitario de Gran Canaria Dr. Negrín: José Pavón Monzo, Nayra Cabrera González. Hospital Universitario Central Asturias: Pablo Herrero Puente, Beatriz María Martínez Bautista. Hospital Universitario de Cabueñes (Gijón): Carmen Elvira Menéndez, Ana Murcia Olagüenaga. Hospital Clínico Universitario Virgen de la Arrixaca: Eva Quero Motto, Nuria Tomás García. Hospital General Universitario Reina Sofía de Murcia: María Consuelo Quesada Martínez, Marta Gómez Gómez. Hospital San Pedro de Logroño: Noemí Ruiz de Lobera. Hospital Clínico Universitario Lozano Blesa: José María Ferreras Amez, Belén Arribas Entrala.
This study has received no financial support of any kind.
The authors declare that they have no conflicts of interest.
Please cite this article as: Miró Ò, Alquézar-Arbé A, Llorens P, Martín-Sánchez FJ, Jiménez S, Martín A, et al. Comparación de las características demográficas y comorbilidad de los pacientes con COVID-19 fallecidos en hospitales españoles, en función de si ingresaron o no en Cuidados Intensivos. Med Intensiva. 2021;45:14–26.