Hemodynamic parameters such as the global end-diastolic volume index (GEDVI) and extravascular lung water index (EVLWI), derived by transpulmonary thermodilution, have gained increasing interest for guiding fluid therapy in critically ill patients. The proposed normal values (680–800ml/m2 for GEDVI and 3–7ml/kg for EVLWI) are based on measurements in healthy individuals and on expert opinion, and are assumed to be suitable for all patients. We analyzed the published data for GEDVI and EVLWI, and investigated the differences between a cohort of septic patients (SEP) and patients undergoing major surgery (SURG), respectively.
MethodsA PubMed literature search for GEDVI, EVLWI or transcardiopulmonary single/double indicator thermodilution was carried out, covering the period from 1990 to 2010.
InterventionMeta-regression analysis was performed to identify any differences between the surgical (SURG) and non-surgical septic groups (SEP).
ResultsData from 1925 patients corresponding to 64 studies were included. On comparing both groups, mean GEDVI was significantly higher by 94ml/m2 (95%CI: [54; 134]) in SEP compared to SURG patients (788ml/m2 95%CI: [762; 816], vs. 694ml/m2, 95%CI: [678; 711], p<0.001). Mean EVLWI also differed significantly by 3.3ml/kg (95%CI: [1.4; 5.2], SURG 7.2ml/kg, 95%CI: [6.9; 7.6] vs. SEP 11.0ml/kg, 95%CI: [9.1; 13.0], p=0.001).
ConclusionsThe published data for GEDVI and EVLWI are heterogeneous, particularly in critically ill patients, and often exceed the proposed normal values derived from healthy individuals. In the group of septic patients, GEDVI and EVLWI were significantly higher than in the group of patients undergoing major surgery. This points to the need for defining different therapeutic targets for different patient populations.
Parámetros hemodinámicos como el índice de volumen diastólico final global (GEDVI) y el índice de agua pulmonar extravascular (EVLWI), obtenidos mediante termodilución transpulmonar, suscitan un interés creciente como guía de la terapia de fluidos en pacientes críticamente enfermos. Los valores normales propuestos (680–800ml/m2 para el GEDVI y 3-7ml/kg para el EVLWI) se basan en mediciones realizadas a individuos sanos y en la opinión de expertos, y se asume que son adecuados para todos los pacientes. Analizamos los datos publicados sobre el GEDVI y el EVLWI e investigamos las diferencias entre una cohorte de pacientes septicémicos (SEP) y pacientes sometidos a cirugía mayor (SURG) respectivamente.
MétodosSe realizó una búsqueda bibliográfica en PubMed de GEDVI, EVLWI o termodilución trasncardiopulmonar de indicador único/doble referida al periodo comprendido entre 1990 y 2010.
IntervencionesSe realizó un análisis de metarregresión para identificar las diferencias entre los grupos quirúrgico (SURG) y no quirúrgico septicémico (SEP).
ResultadosSe incluyeron los datos de 1925 pacientes correspondientes a 64 estudios. Al comparar ambos grupos, el GEDVI medio resultó ser significativamente mayor, con un aumento de 94ml/m2 (IC del 95 %: [54; 134]) en el grupo SEP en comparación con los pacientes SURG (788ml/m2, IC del 95 %: [762; 816], frente a 694 ml/m2, IC del 95 %: [678; 711], p<0,001). El EVLWI medio también presentó una diferencia significativa de 3,3ml/kg (IC del 95 %: [1,4; 5,2], SURG 7,2ml/kg, IC del 95 %: [6,9; 7,6] frente a SEP 11,0ml/kg, IC del 95 %: [9,1;13,0], p=0,001).
ConclusionesLos datos publicados del GEDVI y el EVLWI son heterogéneos, especialmente en pacientes críticamente enfermos, y a menudo superan los valores normales propuestos a partir de individuos sanos. En el grupo de pacientes septicémicos, los índices GEDVI y EVLWI fueron significativamente más altos que en el grupo de pacientes sometido a cirugía mayor. Esto pone de manifiesto la necesidad de definir distintos objetivos terapéuticos para las distintas poblaciones de sujetos.
There is increasing evidence that appropriate hemodynamic management is related to outcome in critically ill patients, both in the operating room and in the intensive care unit.1–3 Reliable assessment of cardiac preload, volume responsiveness, cardiac output (CO) and also indicators for potential fluid overload (extravascular lung water, EVLW) are prerequisites for successful management of hemodynamically unstable critically ill patients.
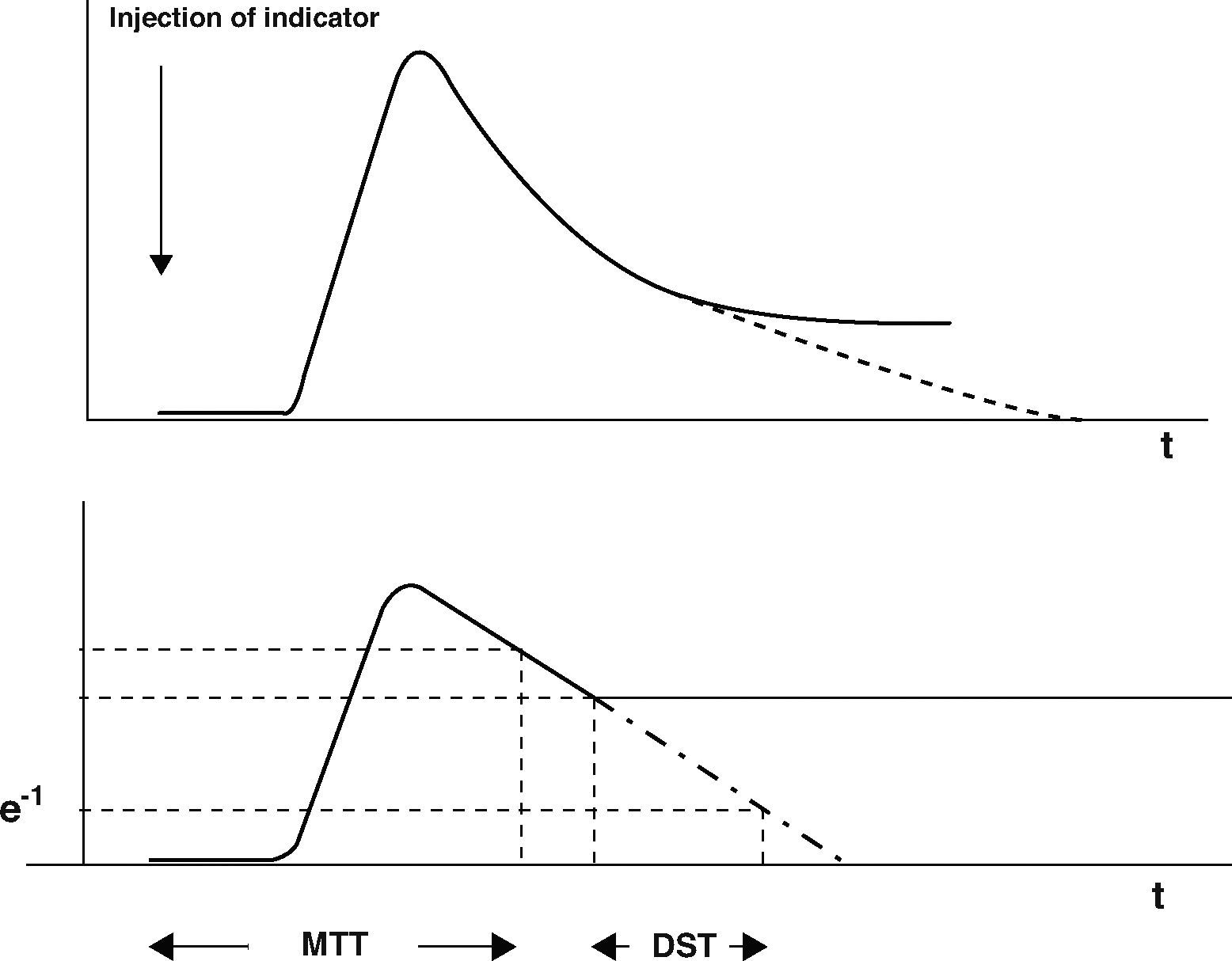
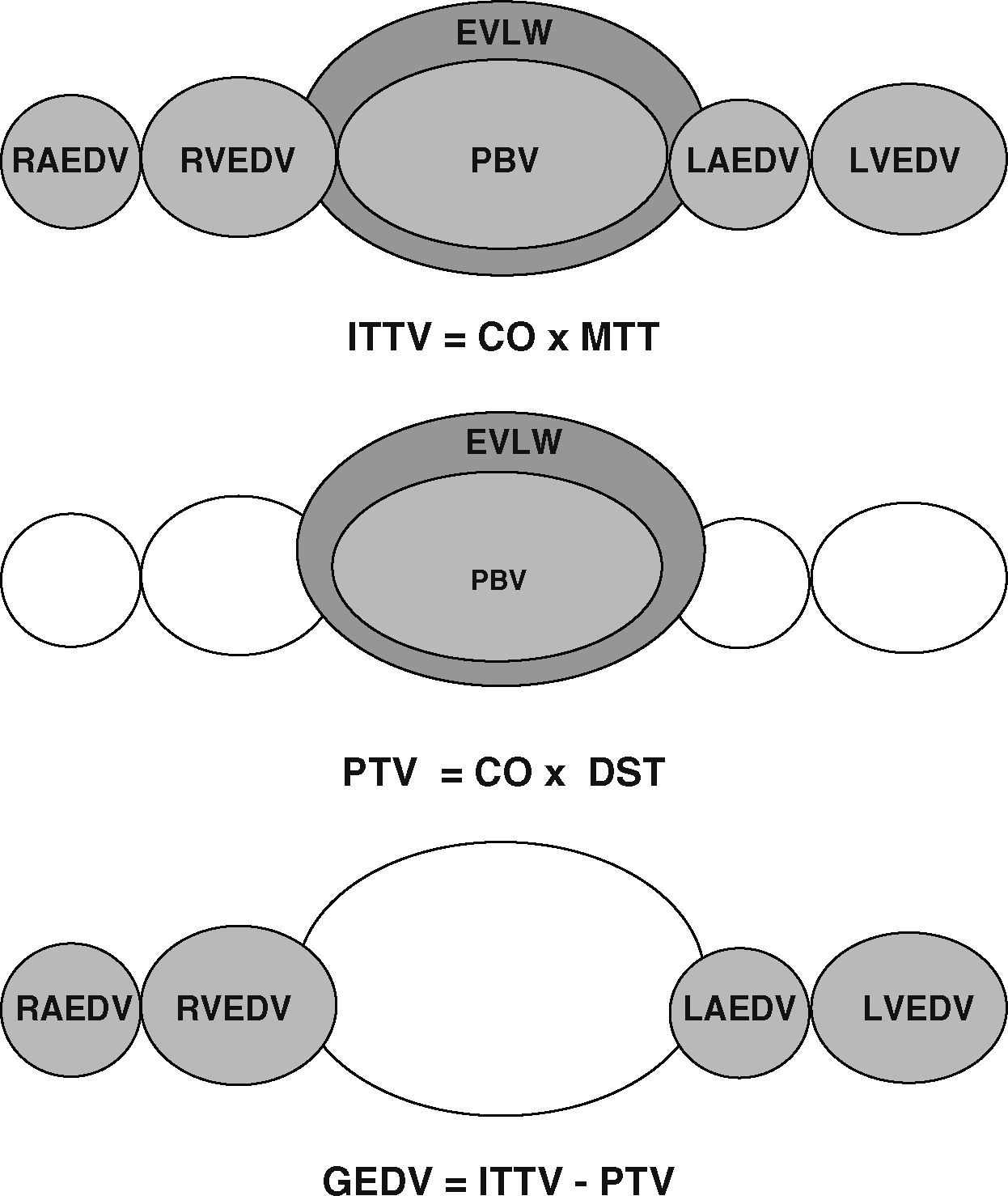
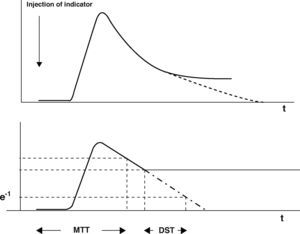
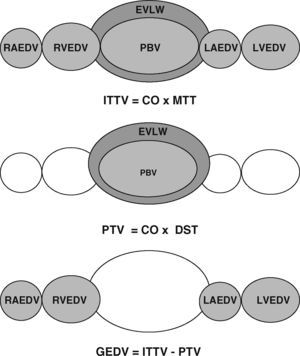
As well as imaging techniques, such as transesophageal echocardiography, thermodilution techniques, and in particular transcardiopulmonary thermodilution, allow accurate assessment of cardiac preload volumes by measuring GEDVI.4–6 For this assessment, cold saline as a freely diffusible indicator is injected randomly throughout the respiratory cycle via a central venous catheter. The mean transit time (MTT) and the exponential downslope time (DST) of the thermal indicator are detected by a thermistor tipped catheter in the femoral artery (Figure 1). ITTV, the intrathoracic thermal volume, is calculated from CO×MTT and the pulmonary thermal volume (PTV) is derived from CO×DST. GEDV is then calculated by subtracting PTV from ITTV (Figure 2). For inter-individual comparability GEDV is then indexed to the patients’ body surface (GEDVI).
The upper curve indicates a thermodilution curve obtained by injection of a cold bolus, showing the temperature over time at the catheter tip. By extrapolation of the curve (dashed line), potential recirculation phenomena are excluded. The lower curve shows the logarithmic extrapolation allowing to define the mean transit time (MTT) and the exponential downslope time (DST) of the indicator.
Assessment of global end-diastolic volume (GEDV) by transcardiopulmonary thermodilution. From top to bottom: first row: the intrathoracic thermal volume (ITTV) is the distribution volume of the thermal indicator, including the right atrium end-diastolic volume (RAEDV), the right ventricle (RVEDV), the pulmonary blood volume (PBV), the extravascular lung water (EVLW), the left atrium (LAEDV) and the left ventricle (LVEDV). It is calculated by multiplying cardiac output (CO) with the mean transit time (MTtT) of the indicator. Second row: the pulmonary thermal volume (PTV) includes the PBV and the EVLW and is assessed by multiplying CO with the exponential decay time (DST) of the thermal indicator. Third row: the GEDV is calculated by subtracting PTV from ITTV.
Hypovolemic patients with decreased cardiac preload present with lower values of GEDVI and are more likely to respond to a volume challenge with a significant increase in CO.6 Because of decreased invasiveness compared to pulmonary artery catheterization, and its greater operator-independency compared to echocardiography, the method has gained increasing acceptance over the last decade among physicians for determining cardiac output and preload and is made commercially available by Pulsion Medical Systems (Munich, Germany).7,8 Also available, the LiDCO plus uses lithium for calibration and provides a reliable CO monitoring (LiDCO, Cambridge, UK).9 Recently, an alternative device (Volume-view, Edwards Life Sciences, Irvine, USA) using basically the same technical approach for measurement of GEDVI as the established PiCCO monitor (PiCCO2, Pulsion Medical Systems, Munich, Germany), has been described as showing equivalent results in an animal model.10
Optimizing preload by volume loading may be limited by excessive fluid retention and the development of tissue edema, especially in the lungs. Here, the degree of tissue edema, i.e. the extravascular lung water (EVLWI), is difficult to quantify but is important information needed to guide therapy.11 Although chest X-ray is widely used to assess the grade of pulmonary edema, there is evidence that it is inadequate for determining fluid overload in the lungs.12 Furthermore the presence of pleural effusions must also be taken into account when interpreting EVLWI.13 Patroniti et al. demonstrated good correlation between lung edema and quantitative computed tomography,14 but this method is associated with high exposure to ionizing radiation and is not available at the bedside, excluding its use as a monitoring device. The EVLWI can be monitored and quantified by indicator dilution techniques and is calculated as the EVLW divided by the predicted body weight.15 EVLWI measured by single transcardiopulmonary thermodilution correlates well with the respective values measured by double indicator techniques16,17 and with human18 and experimental measurements by postmortem gravimetry, representing the experimental gold standard.19–21 Increased EVLWI is associated with poor outcome in critically ill patients.22–24 Furthermore, treatment of Acute Respiratory Distress Syndrome (ARDS) driven by EVLWI has been attributed as being beneficial for outcome in the critically ill.24,25
The use of both GEDVI and EVLWI has also been proposed in treatment algorithms. Their use has pointed towards improved outcome in cardiac surgery patients.26 This led to the inclusion of these parameters into the current treatment guidelines for postoperative cardiac surgery patients.27 The normal values for these parameters are given as 680–800ml/m2 for GEDVI and 3–7ml/kg for EVLWI, which in turn serve as hemodynamic targets.26–28 However, these values are primarily based on initial measurements in healthy individuals and on expert opinion, regardless of patients’ age.
Recently Wolf et al. showed a dependence of GEDV on age, gender, height and weight in a hemodynamically stable patient population, which remained even after indexing the parameter to body surface area.29 These data from non-critically ill patients demonstrate surprising heterogeneity of values. Tagami et al. recently defined a normal EVLWI of 7.3±3.3ml/kg in a human autopsy study showing that the proposed normal values of 3–7ml/kg are possibly not appropriate for most clinical scenarios.18 Additionally it needs to be considered whether these normal values are eligible for all patient groups. For example, differences may be found between critically ill patients suffering from various different diseases and, for instance, short stay surgical patients.
To our knowledge no systematic data analysis of GEDVI and EVLWI values exists between different patient cohorts. As a first step it was therefore necessary to identify the actual reported values of GEDVI and EVLWI in different critically ill populations and secondly to define reasonable treatment goals in these different patients groups.
Therefore we performed a literature search of analyzed, published values for GEDVI and EVLWI in critically ill patients. The aim of our study was to analyze the ranges of published data on GEDVI and EVLWI in adult, critically ill patients, and to explore if differences existed between surgical and non-surgical (predominantly septic) patients.
Materials and methodsWe searched PubMed from January 1990 to April 2010 using the search strategy “transpulmonary/transcardiopulmonary single/double indicator thermodilution” OR “global end-diastolic volume” OR “extravascular lung water”. We restricted the search to studies in adults. Only articles published in English or German were considered. Further information was retrieved through a manual search of references from recent reviews and relevant published original studies.
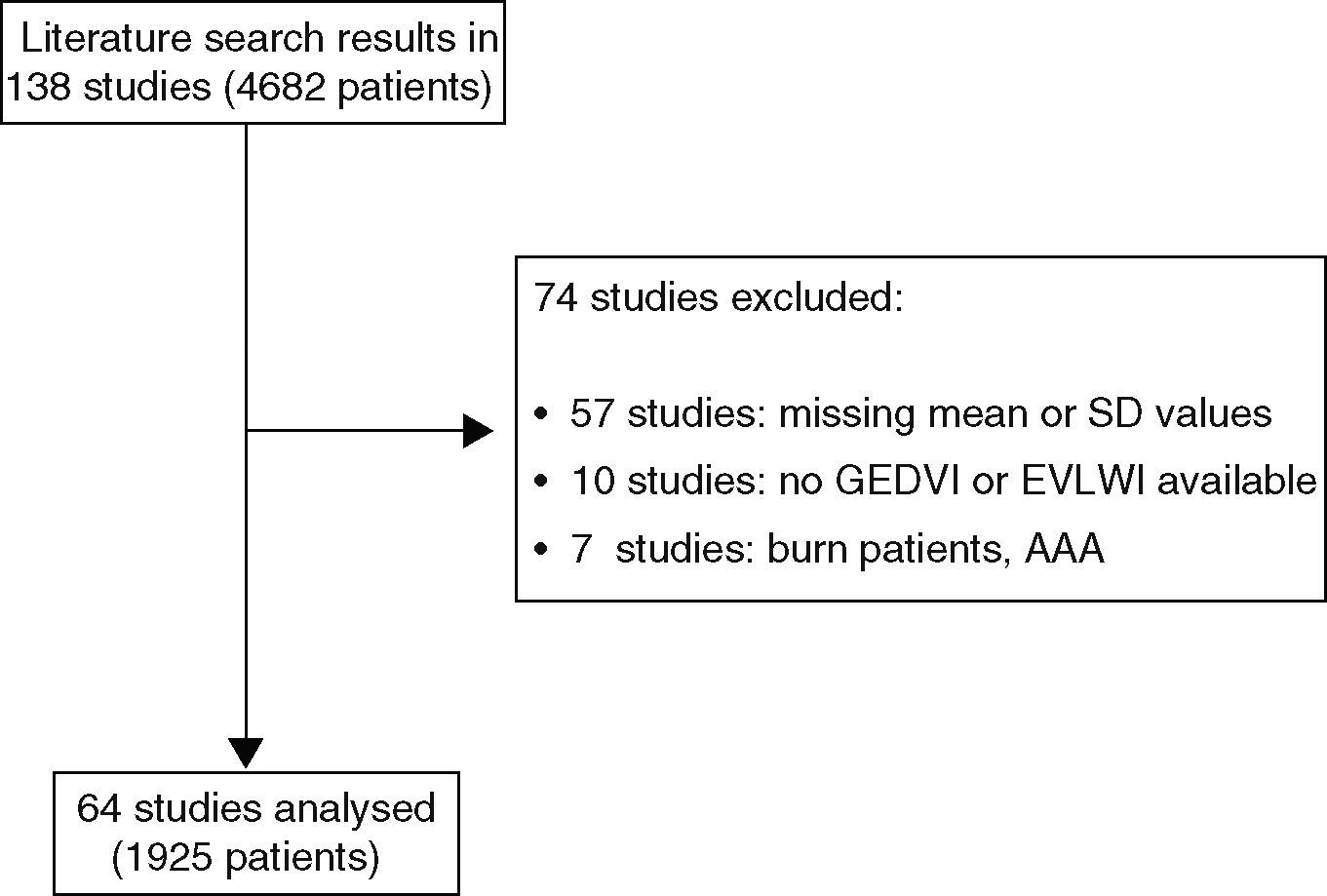
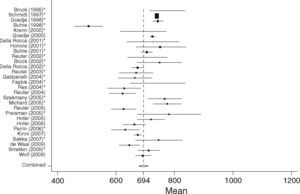
The majority of included studies reported ITBVI instead of GEDVI. For comparability of all analyzed studies GEDVI was determined by calculating ITBVI/1.25, which has been shown to be accurate based on the linear relation between ITBVI and GEDVI.17 In total, 74 studies had to be excluded from the analysis (reasons given in Figure 3). The main reason for exclusion was incomplete data given by the study, such as missing mean or standard deviation values. Furthermore, severe burn patients were also excluded because they have massive capillary leakage and unique volume distribution leading to hypovolemia, and are therefore not comparable to either the surgical or septic patient groups. Patients undergoing aortic surgery were excluded because aortic malformations potentially result in abnormally high indicator distribution volumes. For the same reason studies that used catheterization sites other than the femoral artery were not considered. Furthermore, studies in pediatric patients were excluded.
Meta regression analysis was performed to estimate the difference between the surgical (SURG) and the non-surgical group (SEP), adjusting for heterogeneity within groups.30 All statistical tests were conducted by using Stata 11.0 (StataCorp LP, TX, USA) with a level of significance of 5%.
ResultsWe found 138 articles that included a total of 4682 patients. Data from 1925 patients from 64 studies were included in the final analysis. The majority of patients in the surgical group had underdone cardiac surgery, but several other kinds of major surgery, e.g. abdominal surgery, neurosurgery, were also included in the SURG group. The studies included in the SEP group consisted of critically ill, mechanically ventilated patients predominantly treated for sepsis with accompanying acute lung injury.
Overall the patients showed a wide range of values. GEDVI varied from 378 to 1433ml/m2 and EVLWI from 1 to 46.6ml/kg respectively. After stratification of studies to either SURG or SEP, the groups were analyzed separately and then compared.
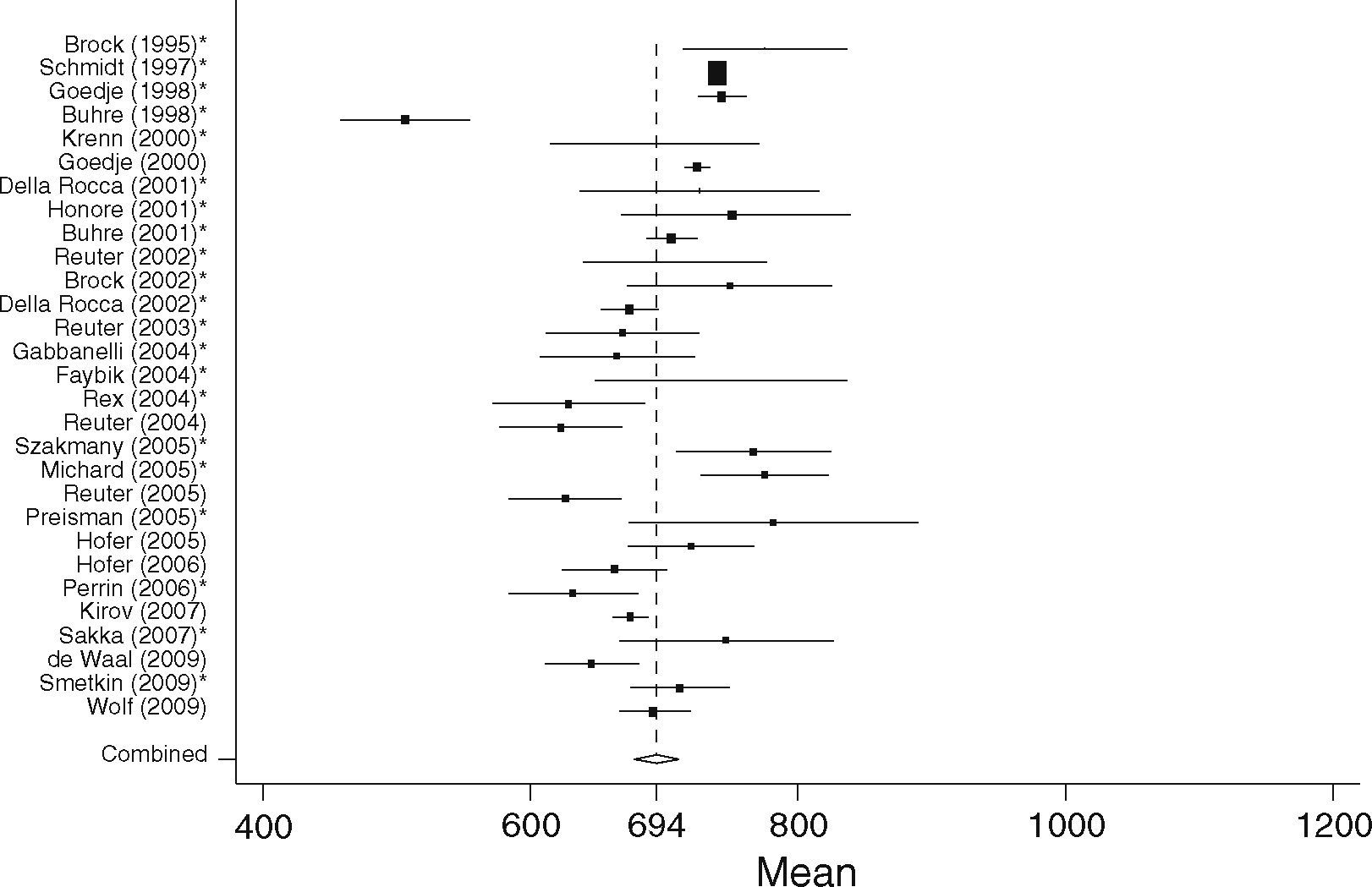
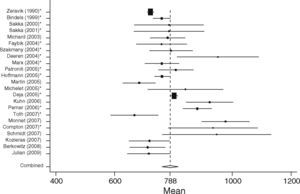
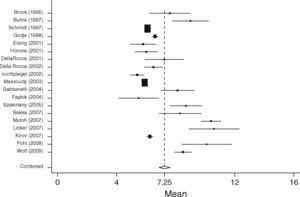
GEDVISurgical patients (SURG)In the surgical group 37 studies with 1127 patients were identified. In total 29 studies including 867 patients fulfilled the inclusion criteria and were statistically analyzed. From the individual papers the lowest mean GEDVI was 506±78ml/m231 and the highest mean GEDVI was 781±234ml/m2 given in a study from Preisman et al., who performed stepwise volume loading in cardiac surgery patients.32 The pooled estimate for the mean value for GEDVI from all papers for the SURG group was 694ml/m2, 95%CI: [677; 711], with the data being significantly heterogeneous (Q=334.6, df=28, p<0.001, see Figure 4).
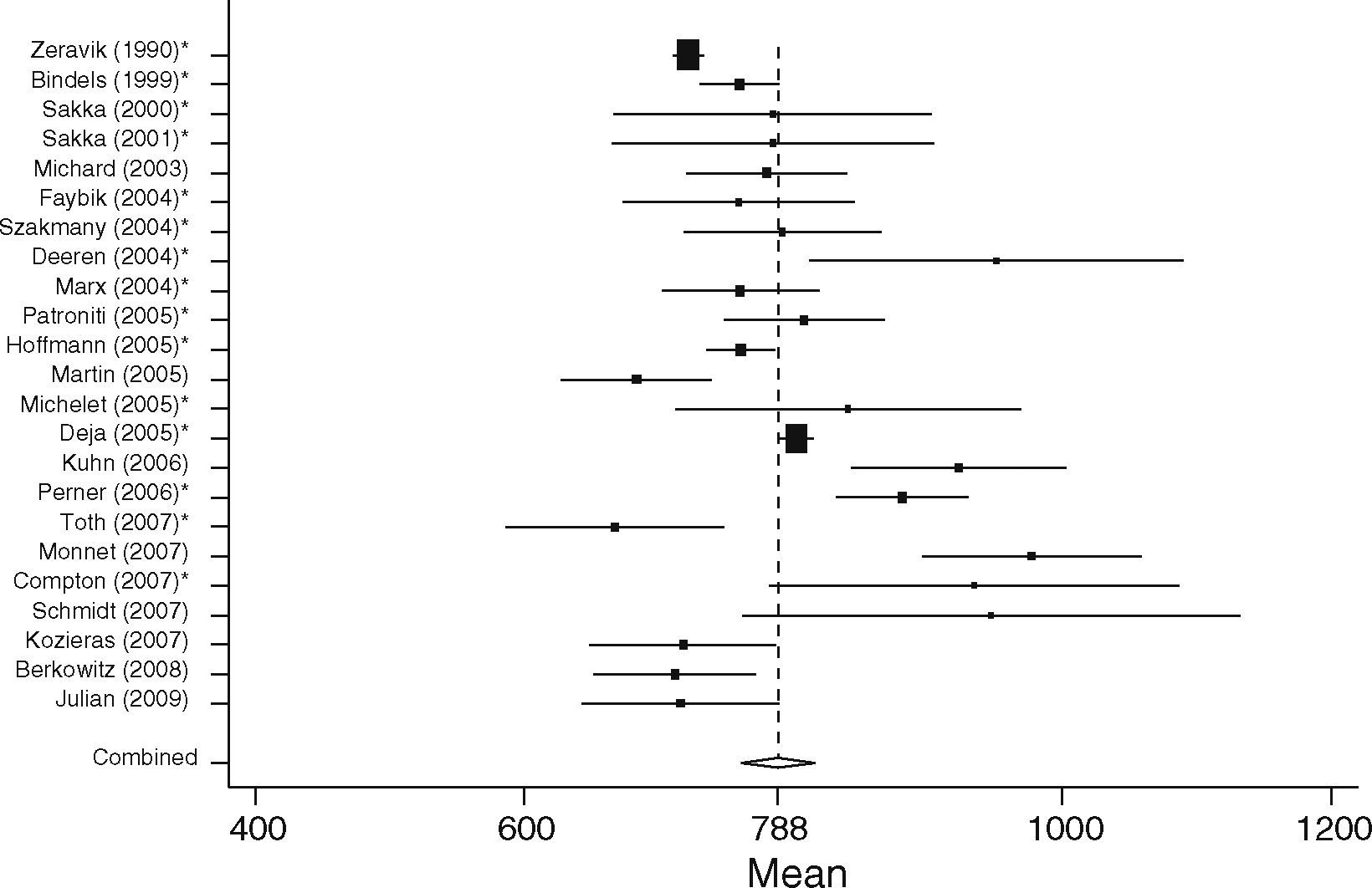
Non-surgical septic patients (SEP)The non-surgical patient group consisted of 701 patients included in 23 studies. Here the lowest mean was 667±177ml/m233 and the highest mean GEDVI was 977±291ml/m2.34 The pooled estimate for the mean value of GEDVI in the SEP group was 788ml/m2, 95%CI: [761; 816]; with data here also significantly heterogeneous (Q=194.7, df=22, p<0.001, Figure 5).
When comparing both groups the mean GEDVI was 94ml/m2 (95%CI: [54; 134]) higher in patients from the SEP group compared to those in the SURG group (788ml/m2 95%CI: [762; 816], vs. 694ml/m2, 95%CI: [678; 711]). Despite the high heterogeneity of the data, statistically significant differences between the groups were found (p<0.001).
In patients undergoing major surgery (SURG) 18 of 29 studies revealed GEDVI values within the given ‘normal range’ of 680–800ml/m2. In non-surgical septic patients GEDVI was outside the proposed ‘normal range’ in 10 of the 23 studies: One study showed data below the lower limit of 680ml/m2 and 9 studies described values above the upper limit of 800ml/m2.
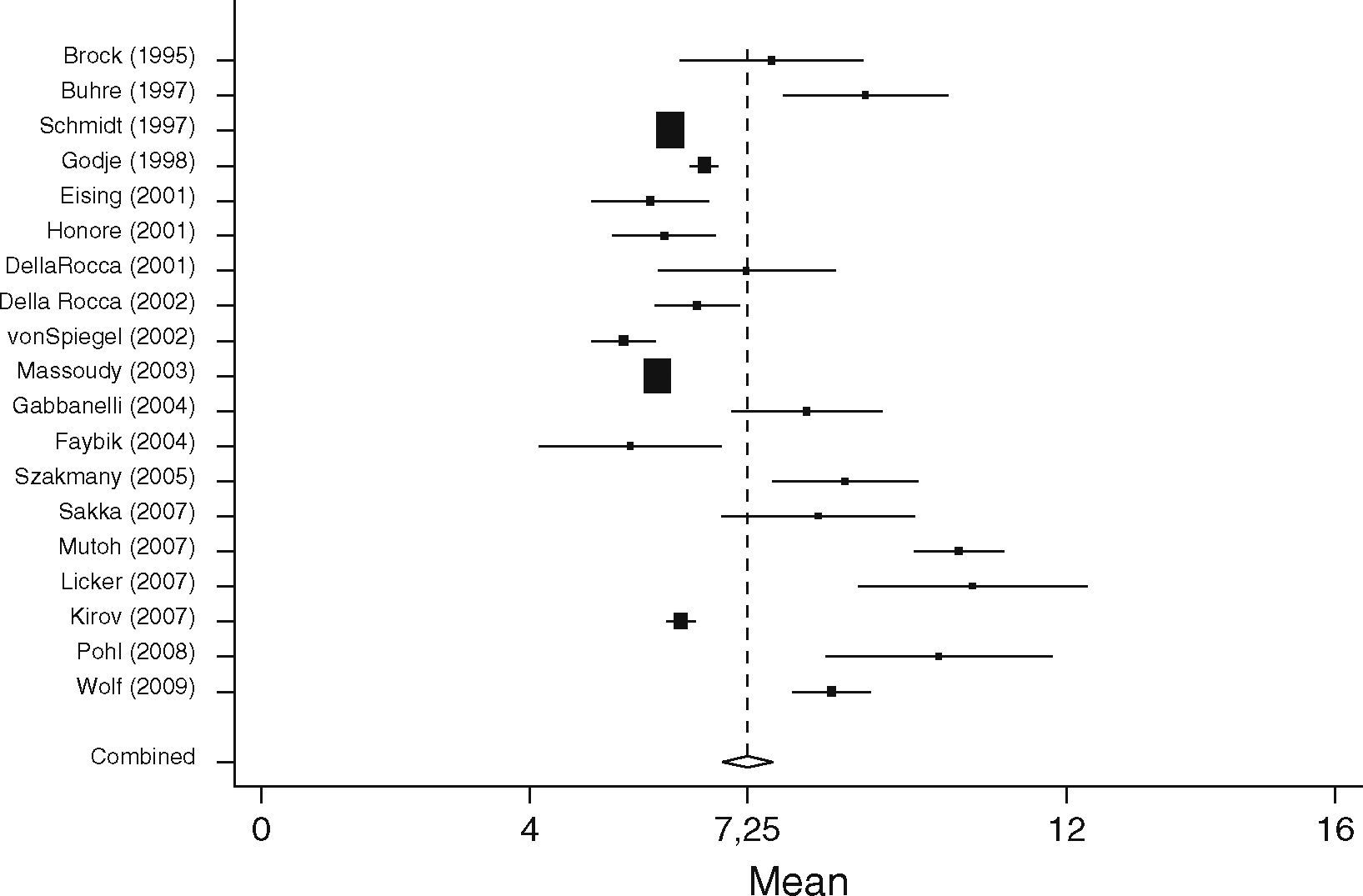
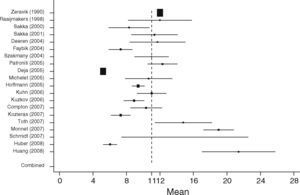
EVLWISurgical patients (SURG)When analyzing EVLWI in the SURG group 19 studies including 687 patients were identified. The lowest mean EVLWI was 5.4±1.1ml/kg.35 The highest mean EVLWI was 10.6±4ml/kg measured in patients undergoing lung resection.36 Here, the included post lung resection values might have led to high values.37,38 Nevertheless, these studies were included in the present analysis because of limited data proving clinical significance of this potential methodological error. The pooled estimate for the mean value of all studies in the surgical patient group was 7.3ml/kg (95%CI: [6.8; 7.6]; heterogeneity: Q=389.4, df=18, p<0.001, Figure 6).
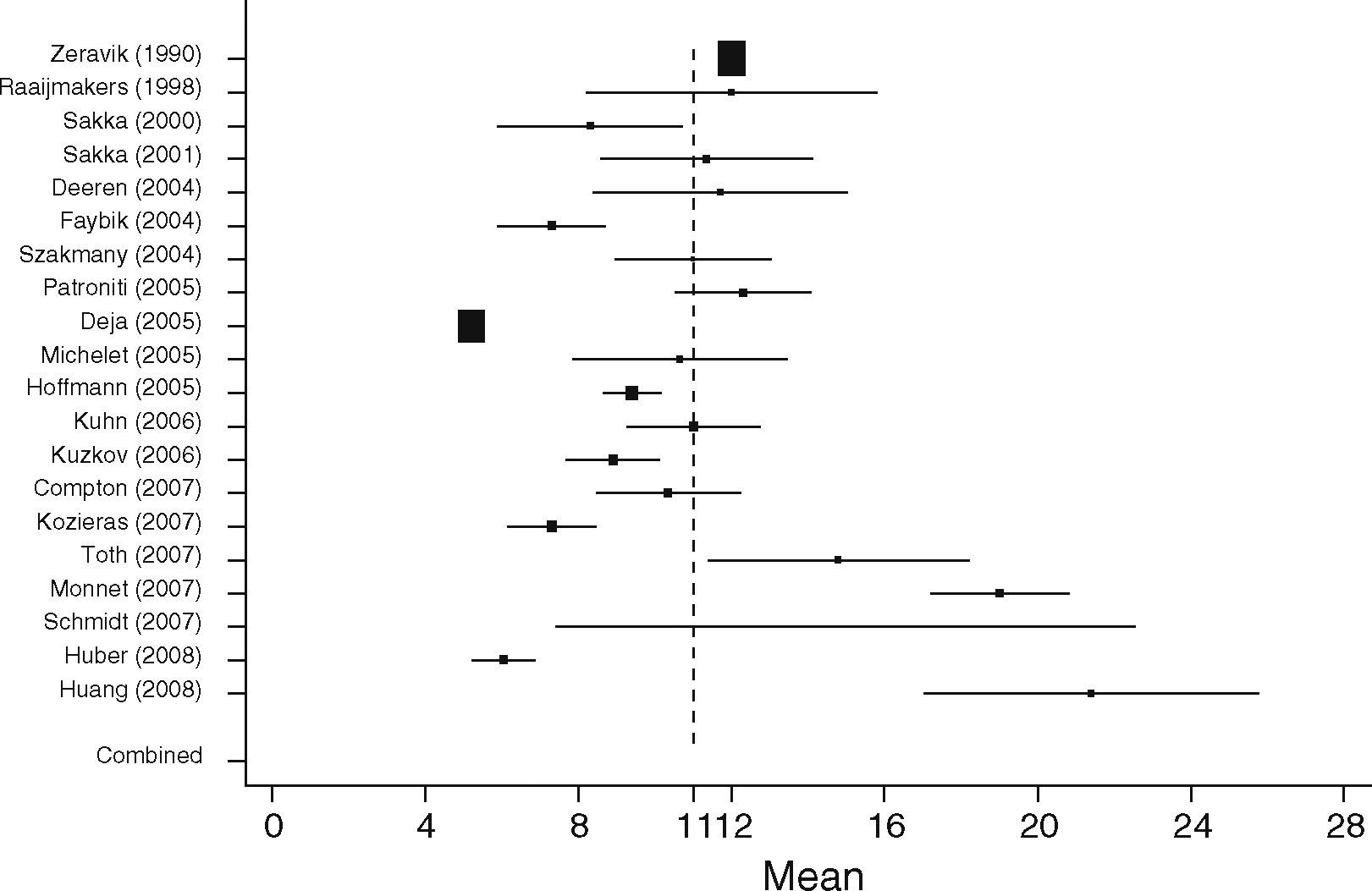
Non-surgical septic patients (SEP)In the SEP group 20 studies with a total of 598 patients were identified. From all studies the highest mean EVLWI was 21.4±10ml/kg and the lowest mean was 5.2±0.5ml/kg.39 The overall pooled estimate for the mean value of EVLWI in the group of medical patients was 11ml/kg, 95%CI: [9.0; 13.0]; heterogeneity: Q=2270.7, df=19, p<0.001 (Figure 7).
When comparing both groups, mean EVLWI differed by 3.3ml/kg (95%CI: [1.4; 5.2], SURG 7.3ml/kg, 95%CI: [6.9; 7.6] vs. SEP 11ml/kg, 95%CI: [9.1; 13.0], p=0.001). In the septic group all studies except one showed EVLWI values above the limit of 7ml/kg (20/21), whereas 9 of the 19 studies including surgical patients gave the normal values of 3–7ml/kg.
DiscussionIn this analysis of 138 articles using transpulmonary thermodilution technique, we found a large variance in data for GEDVI and EVLWI, often exceeding the given ‘normal’ values. Furthermore, data for GEDVI and EVLWI differed significantly between critically ill surgical and septic patients.
For most hemodynamic parameters precise defined values for specific treatment goals are lacking, this applies particularly in critically ill patients. Undoubtedly, the mean arterial pressure (MAP) is the most mentioned and most commonly used parameter in the treatment of circulatory insufficiency.40 The Surviving Sepsis Campaign (SCC) defined a MAP ≥65mmHg and a central venous pressure of 8–12mmHg to be maintained in septic patients.41 But in fact these treatment goals achieve surprisingly low support from other relevant studies. A more critical look at the parameters for preload monitoring shows that there is actually more evidence for the use of volumetric parameters, i.e. GEDVI or left ventricular end diastolic area, and their use in critically ill patients than for filling pressures.42,43
In the present literature analysis 60% of the studies that included surgical patients (SURG group) showed values of GEDVI within the reported normal range of 680–800ml/m2. In the remaining studies data were below the lower range of 680ml/m2 regardless the timing of measurement and the type of operation performed.
The normal value of GEDVI was exceeded more often in the critically ill septic patient group: 30% of the studies gave values above the upper limit of 800ml/m2. The difference of a GEDVI of 94ml/m2 between subgroup analysis between the surgical and septic patients is notable, and in the present meta-analysis this difference reached statistical significance. A high percentage of patients with sepsis show acute and reversible left ventricular dilation resulting in systolic left ventricular dysfunction.44 This acute dilatation in early sepsis and the need for a higher preload volume to maintain sufficient circulation is most probably reflected in these higher values of GEDVI in the group of non-surgical patients. Thus, the proposed range of normal values may not be appropriate in these critically ill patients. It needs to be considered that the given values were based on cardiopulmonary healthy patients and therefore may not be applicable for septic patients, given the high probability that septic patients need a higher GEDVI to optimize cardiac function. Patients’ optimal preload, as expressed by GEDVI, varies between patients’ demographic data, underlying type and severity of disease. Therefore an abnormal GEDVI may be satisfactory for one patient, and a normal GEDVI may be misleading for non-optimal cardiac preload.
This moreover stresses the need to individually determine the patient's optimal preload volume when using volumetric parameters of preload to guide therapy.45 This can either be done by repetitive volume challenges for determining the patients’ ideal cardiac preload, as already proposed46; however, this may potentially lead to repetitive, unnecessary and potentially harmful volume application in patients who are not volume responsive.47 Continuous dynamic indicators of preload such as left ventricular stroke volume variation or arterial pulse pressure variation can help overcome this dilemma, but only in patients on controlled mechanical ventilation without significant arrhythmias.48
For EVLWI, normal values of 3–7ml/kg are proposed. Interestingly, only 50% of the studies in the surgical patient group had values within this normal range. The other 50% were above the upper limit of 7ml/kg. Thus, even in this population of surgical patients without long-term intensive care treatment and supposedly without clinically relevant pulmonary edema half of the EVLWI values exceeded the proposed normal value. This finding is noteworthy as it may point towards potential fluid overload for a significant portion of surgical patients. However in the studies including predominantly sepsis patients all mean values for EVLWI were above this upper limit of 7ml/kg. These studies also revealed a significantly higher EVLWI when compared to the studies performed in surgical patients. This difference is expected, because mechanically ventilated patients in intensive care units suffering from systemic inflammation frequently demonstrate changes in pulmonary permeability.49 Therefore the upper limit for EVLWI of 7ml/kg almost always exceeded in critically ill patients. This may lead to the concept that maybe the established ideal goal of 7ml/kg is too conservative, and perhaps leads to potentially harmful fluid restriction in patients with impaired organ perfusion. Although it is doubtful that patients will remain under resuscitated initially because of a low EVLWI, a high EVLWI above 10–12ml/kg remains a reasonable trigger to start late conservative fluid management or late goal directed fluid removal as was recently shown.24,50 This holds true particularly when evaluating the increasing evidence that the level of EVLWI correlates with outcome in critically ill patients, promoting the definition of therapeutic goals in this group of patients. However, these goals should then be in line with these findings. Sakka et al. reported a significant increase in mortality in patients with severe sepsis, when EVLWI exceeded 14ml/kg.25 Thus, for patients with sepsis, values of up to 10–12ml/kg may be tolerable, although more data are needed in this regard.22,51 Just recently, Phillips et al. showed in critically ill patients the prognostic value of a rise in EVLWI to predict acute lung injury. They also suggested of a trigger point of not less than 10ml/kg.52,53 Therefore treatment goals of 3–7ml/kg as proposed as the normal values may not be appropriate in particular in this group of patients. In summary however, combining measurements of GEDVI and EVLWI with volume loading enables balanced volume therapy, i.e. optimized stroke volume and fluid overload avoidance.
Furthermore, in surgical patients in whom duration of ventilation is normally shorter than in patients admitted to the intensive care unit with severe sepsis, half of the studies included in the present data analysis described values of EVLWI above the upper limit of normal EVLWI of 7ml/kg. This might be explained by perioperative stress and inflammation due to the surgical procedure, but in patients lacking pulmonary alterations it remains notable. This also points towards the fact that the proposed normal range for EVLWI seems only suitable for healthy volunteers and are hardly ever seen in critically ill patients or in patients undergoing moderate to major surgical procedures. These assumptions were confirmed by Tagami et al. in a human autopsy study where they defined a normal EVLWI value of 7.4±3.3ml/kg, already slightly above the given normal values of 3–7ml/kg.18
Several limitations to the present data analysis need to be highlighted. We included all studies found by an extended literature search which documented GEDVI and/or EVLWI and which could be allocated to either a group of surgical patient's monitored perioperatively or to a group of non-surgical, septic patients. Even though most studies could clearly be assigned to either patient group, definition of these groups was performed arbitrarily, and contamination cannot be ruled out. More subgroups, such as burn patients, could have been created, but none would have obtained a statistically relevant number of patients. Heterogeneity of patients between studies, number of patients per study, timing and number of measurements performed, treatment of patients such as use of vasopressors, inotropes or fluid bolus, as well as type of operation or cause of sepsis may also limit the conclusions of this study. Due to the high heterogeneity our results have to be interpreted with caution, but we believe that the statistical significance reached between both groups helps to integrate the data into the clinical management of such patients. We were not able to obtain individual data to re-analyze different thresholds for EVLWI or GEDVI in relation to outcome, nor were we able to calculate corrected GEDVI according to the global ejection fraction (GEF) since this recently has been shown to correlate better with the true preload status especially in patients with low GEF and high GEDVI.54
ConclusionsWe conclude that the published values for GEDVI and hemodynamics derived by transcardiopulmonary thermodilution may be misleading under certain clinical circumstances. The proposed values are based on normal values for healthy volunteers and are therefore not directly applicable for critically ill patients. Septic cardiac impairment, i.e. ventricular dilation may be part of the reason why cardiac filling volumes (GEDVI) are often elevated in septic patients. We assume that an individual volume loading approach would be more likely to optimize cardiac preload, even though the actual GEDVI may often be above the upper limit of given values. Our findings show significant differences in GEDVI between surgical and septic patients underlining this assumption.
The normal values given for EVLWI are unlikely to be found in perioperative surgical patients and are almost never seen in critically ill patients with sepsis. Using the proposed normal values of EVLWI as therapeutic targets for septic patients seems therefore questionable, and modifications oriented to values associated with decreased patients’ outcome would appear be more reasonable.
Conflict of interestDaniel A. Reuter and Manu LNG Malbrain are members of the Pulsion Medical advisory board (Pulsion Medical Systems, Munich, Germany).