To gather all published information about the stability of drugs commonly used in Intensive Care Units (ICU); evaluate the methodology of published data; and generate a compatibility table.
Design(i) A systematic review was conducted searching the following databases: Medline, Stabilis, Handbook of Injectable Drugs and Micromedex. Articles published from 1990 to 2017 in English, Spanish and French were included. (ii) Article quality was analyzed according to the stability studies practice guidelines. (iii) A compatibility table was produced with data for 44 binary combinations of drugs frequently used in the ICU.
ScopeSpanish and international hospital ICU.
ResultsThe systematic review included 29 studies (27 originals, 2 reviews). None of the included studies followed all the methodological requirements. However, 93% guaranteed correct reproducibility. Accordingly, drug stability knowledge was available for 50.3% of the studied admixtures, in which 77.1% of the binary combinations proved compatible and 16.8% proved incompatible.
ConclusionsThis review provides new reliable evidence about the physicochemical stability of drugs commonly used in the critical care setting. The study contributes to the safe administration of intravenous drugs in critical patients with a view to avoiding adverse events in this frail population.
Recopilar la información publicada sobre estabilidad de los fármacos usados en el paciente crítico, evaluar la calidad de los datos publicados y generar una tabla de compatibilidad con información actualizada.
Diseño1) Se realizó una búsqueda sistemática en las bases de datos Medline, Stabilis, Handbook on Injectable Drugs y Micromedex, para completar y actualizar la información disponible. Se incluyeron los estudios publicados entre 1990 y 2017 redactados en inglés, español y francés; 2) se analizó la calidad de los artículos según los criterios indicados en las guías de práctica para estudios de estabilidad; 3) se construyó una tabla de compatibilidades con los datos hallados para las combinaciones binarias de 44 fármacos de uso frecuente en unidades de cuidados intensivos (UCI).
ÁmbitoUCI de hospitales españoles e internacionales.
ResultadosLa revisión sistemática incluyó 29 artículos (27 originales y 2 revisiones). Ningún estudio cumplió todos los criterios de calidad establecidos, aunque el 93% garantizaba una correcta reproducibilidad. La tabla final aporta datos de compatibilidad fisicoquímica de 475 de las 945 combinaciones posibles (50,3%), de las cuales 366 (77,1%) son compatibles y 80 (16,8%) son incompatibles.
ConclusionesSe proporciona una actualización de las compatibilidades entre los fármacos habitualmente empleados en las UCI, con la intención de contribuir a la administración segura de medicamentos en pacientes críticos.
Patients admitted to intensive care units (ICU) often require the IV administration of several drugs. Vasoactive drugs, analgesics, and sedatives are among the most widely used therapeutic groups and are usually administered in continuous infusion.
According to the systematic review conducted by Moyen et al. there is an average 1.7 errors/day associated with the process of drug administration in the ICU setting.1 On the other hand, the data reported by Merino et al. in a study conducted among Spanish hospital ICUs are a little better (1.13 medication errors for every 100 patients/day).2 Even so, medication errors are common in ICUs and require care from healthcare providers to minimize them.
Errors in the administration of drugs in ICUs are due to several factors: the use of high-risk drugs (vasoactive drugs, inotropes, sedatives, etc.) often administered in low doses due to their high drug strength, requiring dilution and a prior assessment to their administration. Another factor is the prescription of doses in different units of measurement or the high number of drugs used with each patient. Although it is an important advance with regard to safety, the use of intelligent infusion pumps has been associated with an important number or medication errors due to programming issues.3
The combination of these risk factors increases the chances of making mistakes in the most vulnerable patients due to their severity. Critically ill patients often have limited venous accesses. This means that different drugs are delivered using the same route of administration, which increases the risks involved when mixing incompatible drugs. The mix of incompatible drugs is a medication error that can have serious consequences for the patient such as therapeutic failures, micro-embolism or toxicity.4
The Y-site infusion of 2 drugs requires both drugs to be physically compatible.5 This coadministration occurs when mixing drugs in a 1:1 ratio and in the absence of visible signs of incompatibility like precipitation or change in color. On the other hand, for the safe coadministration of 2 drugs in the same diluent, the mix needs to be chemically stable. This means prior confirmation is needed that no significant change has occurred in the concentration of either one of the drugs present in the mix.6
Standardizing the concentration of infusion solutions is one of the most useful measures to prevent medication errors in the ICU setting, especially in high-risk drugs due to their potential to cause severe damage and because they have the highest incidence of medication errors.
Another highly recommended measure for the safe administration of drugs is having reliable information available on drug compatibility when administering common drugs in critically ill patients. However, information on drug compatibility is scarce and, on many occasions, difficult to interpret due to the different concentrations used, the lack of information on the assessment techniques used or the suspicious technical quality of the sources. The lack of information on the safe mix of 2 drugs creates problems in the daily work of ICU nursing teams. Added to the risk of complications associated to the administration of 2 incompatible molecules, this lack of information can make the nurse have to look for new venous accesses to administer the drugs separately whichincreases the risk of infectious or thromboembolic complications.
The goal of this review is to gather the information published on the physical and chemical compatibility of the most commonly used drugs at an ICU when infused through the same line via a Y-site. Also, to assess the quality of the information published and generate a compatibility chart with reliable and updated information to improve safety in the administration of drugs to critically ill patients.
MethodologySearch strategyA systematic search on Medline, Stabilis, Handbook on Injectable Drugs, and Micromedex databases was conducted for the identification of original papers, review articles and meta-analyses on the physical and chemical compatibility of drugs. Due to their clinical approach and lack of methodology to determine physical and chemical stability, case studies were discarded. The reviews published by Kanji et al. and López-Cabezas et al.5,7 were used as a reference point. Search focused on drug combinations on which these authors had no information or had not looked for information. The years of publication of the studies went from the1990s until December 2017 and the languages included were English, Spanish, and French. The search strategy consisted of using multiple terms describing the information of interest to combine them with the Boolean operator “OR” followed by refine search using the “AND” operator. The terms used were physical compatibility, drug stability, y-site, y-injection, intravenous drug, plus the names and synonyms of the drugs of interest.
The drugs used in the review are routinely used in the ICU setting are often administered by continuous infusion. The concentrations used as a reference are the ones standardized in our center7 for these drugs and are consistent with the ones commonly used in most ICUs (Table 1). All information on compatibility found for a certain molecule about a different concentration interval is shown in Table 2. The reference search process for each drug was conducted concurrently by 2 independent researchers.
Study drugs and concentrations used as reference for the bibliographic search.
| Drug | Standard concentration | Drug | Standard concentration |
|---|---|---|---|
| Adrenaline | 40mcg/mL | Isoproterenol | 4mcg/mL |
| Amiodarone | 3.6mg/mL | Ketamine | 50mg/mL |
| Argatroban | 1mg/mL | Labetalol | 2mg/mL |
| Bicarbonate | 1mmol/L | Magnesium sulfate | 15mg/mL |
| Calcium chloride | 10mg/mL | Meropenem | 30mg/mL |
| Calcium gluconate | 10mg/mL | Methadone | 0.2mg/mL |
| Ceftazidime | 24mg/mL | Midazolam | 4mg/mL |
| Cisatracurium | 2mg/mL | Milrinone | 0.2mg/mL |
| Clonidine | 7.5mcg/mL | N-acetylcysteine | 50mg/mL |
| Morphine chloride | 1mg/mL | Naloxone | 8mcg/mL |
| Dexmedetomidine | 4mcg/mL | Nitroglycerin | 0.2mg/mL |
| Diltiazem | 1mg/mL | Nitroprusside | 0.2mg/mL |
| Dobutamine | 8mg/mL | Noradrenaline | 0.32mg/mL |
| Dopamine | 8mg/mL | Pantoprazole | 0.32mg/mL |
| Esomeprazole | 0.32mg/mL | Piperacillin-tazobactam | 64mg/mL |
| Phenylephrine | 0.2mg/mL | Potassium chloride | 120mEq/L |
| Fentanyl | 30mcg/mL | Propofol | 10mg/mL |
| Flumazenil | 40mcg/mL | Remifentanil | 20mcg/mL |
| Furosemide | 2mg/mL | Somatostatin | 24mcg/mL |
| Sodium heparin | 50IU/mL | Vecuronium | 0.2mg/mL |
| Insulin | 1IU/mL | Verapamil | 0.1mg/mL |
Combinations of physical and chemically compatible drugs with concentrations below the reference mark.
| Drug#1 | Maximum compatible concentration | Drug#2 | Maximum compatible concentration |
|---|---|---|---|
| Adrenaline | 32mcg/mL | Pantoprazole | 0.8mg/mL |
| 2mcg/mL | Verapamil | 0.08mg/mL | |
| Amiodarone | 4mg/mL | Phenylephrine | 0.04mg/mL |
| 6mg/mL | Furosemide | 1mg/mL | |
| 15mg/mL | Nitroprusside | 0.3mg/mL | |
| Calcium chloride | 4mg/mL | Dobutamine | 4mg/mL |
| Calcium gluconate | 4mg/mL | Dobutamine | 4mg/mL |
| Ceftazidime | 120mg/mL | Dobutamine | 1mg/mL |
| 120mg/mL | Dopamine | 0.4mg/mL | |
| 125mg/mL | Ketamine | 10mg/mL | |
| Dobutamine | 1mg/mL | Heparin | 50IU/mL |
| 4mg/mL | Magnesium sulfate | 40mg/mL | |
| 4mg/mL | Potassium chloride | 60mEq/L | |
| Dopamine | 3.2mg/mL | Midazolam | 2mg/mL |
| Fentanyl | 12.5mcg/mL | Remifentanil | 0.25mg/mL |
| Heparin | 20IU/mL | Verapamil | 0.08mg/mL |
| Isoproterenol | 4mcg/mL | Magnesium sulfate | 1mg/mL |
| 200mcg/mL | Potassium chloride | 40mEq/L | |
| 4mcg/mL | Vecuronium | 0.1mg/mL | |
| 10mcg/mL | Verapamil | 0.08mg/mL | |
| Meropenem | 22mg/mL | Potassium chloride | 40mEq/L |
| Naloxone | 0.8mcg/mL | Verapamil | 0.08mg/mL |
| Nitroglycerin | 0.1mg/mL | Verapamil | 0.08mg/mL |
| Nitroprusside | 0.2mg/mL | Vecuronium | 0.1mg/mL |
| 0.1mg/mL | Verapamil | 0.08mg/mL | |
| Noradrenaline | 0.008mg/mL | Verapamil | 0.08mg/mL |
| Piperacillin-tazobactam | 40mg/mL | Dexmedetomidine | 4mcg/mL |
| 40mg/mL | Remifentanil | 250mcg/mL | |
| Potassium chloride | 100mEq/L | Remifentanil | 250mcg/mL |
It is consistent with the gray boxes specified as I/C as shown in Fig. 2.
After the reference search, 2 independent reviewers assessed the quality of the studies using a peer-review process. This review was conducted following quality criteria based on the opinion of experts and following clinical practice guidelines8–11:
- 1.
Study reproducibility: description of active ingredient and diluent, study conditions and methodology.
- 2.
Number of tests run (at least in triplicate).
- 3.
Times elapsed while taking the samples in the stability analysis: a 5-time sample time period is recommended including a sample time of 0.
- 4.
Studies conducted to assess the stability of the mix: (a) transparency: for visible particles, observation with a matt black panel, automatic particle count or turbidimetry; for subvisible particles, use of optic microscopy, spectrophotometry or turbidimetry; (b) change in color: visual inspection or spectrophotometry; (c) gas formation: visual inspection; (d) pH; and (e) chemical stability: measurement of the variation of the concentration of the 2 drugs.
A chart was created with all the possible combinations of the drugs of interest. Boxes were named with a “C” if the mix was compatible, with an “I” if incompatible and with “I/C” if stability depended on special conditions. The drug combination with no compatibility data were left unchecked.
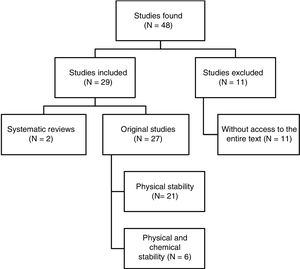
ResultsPerformance of reference searchA total of 48 papers were identified. Fig. 1 shows the selection process. Out of the 29 papers included in the review, 4 were written in Spanish, 3 in French, and 22 in English. Regarding the dates of publication, 8 papers were published between 1990 and 1999, 10 between 2000 and 2009, and the remaining 11 papers were published between 2010 and 2017.
Quality of the studies foundNone of the papers studied met all of the quality criteria established in this review. However, 93% of the papers described the conditions and methodology of the study with enough detail to guarantee its reproducibility.
Tests were run in triplicate only in 26% of the cases. On the contrary, 81% of the studies followed the recommendation of taking samples at time 0, although only 10 obtained a sample in 5 different times.
Regarding the trials conducted to assess the stability of the samples, all studies assessed transparency while 93% of studies reported a change in color through visual inspection. Other methods were used in 16 studies (59%) to see subvisible particles. 67% of the studies assessed gas formation, and only 12 measured pH changes in time. Only 6 studies assessed the chemical stability of the mixes being high-resolution liquid chromatography the method used in 5 studies to measure the concentration of the active ingredients of the mix.
The results on this section are summarized in Table 3.
Summary of the quality criteria of the papers published.
| Quality indicator | Number of studies (%) | References |
|---|---|---|
| Assessment of precipitate formation | 27 (100) | 12,13,16–40 |
| Assessment of change in color | 25 (93) | 12,13,16–27,30–40 |
| Measurement of pH change | 12 (44) | 12,13,16,18,19,22,26,27,29,38,39 |
| Assessment of gas formation | 18 (67) | 12,13,17,19–21,24–26,31,33–40 |
| Analysis performed in triplicate | 7 (26) | 18,21,22,29,34,35,38 |
| Description of the methodology used (includes number and frequency of observations and study conditions) | 24 (89) | 12,13,17–24,26–33,35–40 |
| Description of diluents of all study drugs | 21 (78) | 13,17–29,33,35–40 |
| Description of the material of the study recipients | 22 (81) | 12,13,17–31,33,36–39 |
| Chemical stability | 6 (22) | 16,18,19,22,29,38 |
Forty-four drugs used in continuous perfusion at the ICU setting were selected including a solution for parenteral nutrition with and without lipids and 3 beta-lactam antibiotics. The compatibility of these is shown in Fig. 2. The data obtained by the reviews conducted by Kanji et al. and López-Cabezas et al. provided compatibility information on 393 out of 945 possible combinations.5,7 After completing the systematic review, new stability data for 82 drug combinations were added. The new findings revealed 29 compatible combinations, 27 incompatible combinations, and 26 compatible combinations in specific conditions. Therefore, the final table shows the compatibility data of 475 out of 945 possible combinations of 2 drugs (50.3%). Of these, 366 are compatible (77.1%), 80 are incompatible (16.8%), and 29 are compatible in specific conditions (6.1%) as shown in Table 2.
DiscussionMaking sure that the use of drugs is safe is one of the main commitments made by healthcare providers with their patients. In the ICU setting and given the huge amount of IV drugs administered and the patients’ limited number of routes of administration, this safety is sometimes compromised due to the risks involved when co-administering incompatible drugs in especially vulnerable patients.
Online databases like Stabilis 4.0 are very useful to look for information on drug compatibility. However, the personnel administering the drugs finds charts much more useful because they can quickly look at the information they need at a given time. This is especially interesting in urgent situations when any delays caused by the healthcare providers can have consequences in the patient.
This review focused on analyzing the physical and chemical compatibility of the IV drugs most commonly used through Y-site infusion in the ICU setting and summarizing the information obtained in a double-entry chart. Physical compatibility studies are the most common of all because they are easy to conduct. Chemical stability studies, however, are not because they require more sophisticated analytical techniques to determine the initial and final concentration of drugs.
Despite this, the number of drug combinations studied is still insufficient. As Fig. 2 shows we could not find any information on the physical and chemical compatibility of all the combinations suggested; for instance, in the case of flumazenil and piperacillin-tazobactam we could only determine stability with 4 drugs and in both cases the 39 remaining combinations remained with no information.
Even if we took all the possible combinations suggested into consideration and added the new data found, we would still have zero information on the physical and chemical compatibility of 470 combinations. This means that we only have data available for 50.3% of all the possible combinations suggested.
The most problematic combinations regarding incompatibility are drugs whose stability is closely linked to the pH interval; this is the case with sodium bicarbonate, furosemide or pantoprazole. Furosemide, for example, requires a basic pH to guarantee the stability of the molecule in solution, which is why the mix with acid drugs (pH<4) causes turbidity and precipitation.12
The presence of adjuvants in the pharmaceutical formulation, the concentration and exposure to extreme temperatures or luminosity are other factors associated with drug incompatibility.13 There are times when a given drug combination can be stable in a certain diluent and incompatible in another; for instance, dopamine is only compatible with amiodarone when both are dissolved in glycosylated serum at 5% because the latter in unstable in saline solutions at 0.9%. Thus, if this allegedly compatible mix is performed in physiological serum, a loss of concentration of amiodarone can occur with the corresponding risk of lack of therapeutic response.
On the other hand, in many cases, the quality of the studies published so far can be better. It would be good to have greater uniformity in the quality standards of this type of studies. For example, even though the pH is a critical factor in the stability of drugs in solution, it was only verified in 12 of the 27 papers. Similarly, turbidimetry or microscopy—more accurate techniques than visual observation for the detection of particles and changes in color—are underused. Over the last few years, several experts have published guidelines for the design of drug stability studies.8–11 We can only hope that this will improve the overall quality of this type of studies in the future.
Former authors have published reviews of these characteristics. For instance, Flamein et al.14 studied this problem in neonatal ICUs; Knudsen et al.15 shed light on the compatibility of analgesics and sedatives. Our review is based on the previous work done by Kanji et al.5 in Canada and López-Cabezas.7 in Spain. It has been completed with the new information available on drugs in our setting and data on the most widely used concentrations of drugs.
Overall, we found information on 82 new drug combinations from 27 different references including combinations of 3 beta-lactam antibiotics (ceftazidime, meropenem, and piperacillin-tazobactam) widely used at the ICU setting. Over the last few years the pharmacokinetic advantages of a prolonged perfusion route of administration of these 3 antibiotics have been confirmed.16–19
Perfusions at drug concentrations that exceed the usual ones are often used in the critically ill patient. In this sense, we could not find data on all drug combinations regarding the high concentrations used in the ICU setting (Table 1); however, in some cases, we did obtain information on lower concentrations than the ones reported in this review. These cases are shown on the compatibility chart (Fig. 2) as conditioned compatibility (I/C), that is, that the combination had been studied at a concentration different from the standard one.
The stability data reported in this review cannot be generalized to other drug combinations or concentrations different from the ones described. Also, the information provided is in regard to 2 drug combinations, and incompatibilities may be present with>2 drug combinations at a time, which is highly not advisable. Nevertheless, the drugs and concentrations selected are the most widely used in the adult ICUs of most hospitals.
Until we have new and better compatibility studies that shed some light on this issue, this review can be an easy-to-read update on the evidence available on the compatibility of the drugs most commonly used at the ICU setting. Its goal is to contribute to the safe administration of drugs to patients who can face the consequences of greater severity due to their frailty.
Authors’ contributionGenís Castells Lao: study design and idea, data mining, analysis and interpretation of data; paper draft or critical review of the intellectual material; and final approval of this version.
Montse Rodríguez Reyes: study design and idea, data mining, analysis and interpretation of data; paper draft or critical review of the intellectual material; and final approval of this version.
Judit Roura Turet: data mining, analysis and interpretation of data; paper draft or critical review of the intellectual material; and final approval of this version.
Marta Prat Dot: data mining, analysis and interpretation of data; paper draft or critical review of the intellectual material; and final approval of this version.
Dolors Soy Muner: study design and idea; paper draft or critical review of the intellectual material; and final approval of this version.
Carmen López Cabezas: study design and idea, data mining, analysis and interpretation of data; paper draft or critical review of the intellectual material; and final approval of this version.
Conflicts of interestThe authors declared no conflicts of interest whatsoever.
Please cite this article as: Castells Lao G, Rodríguez Reyes M, Roura Turet J, Prat Dot M, Soy Muner D, López Cabezas C. Compatibilidad de los fármacos administrados en «Y» en las unidades de cuidados intensivos: revisión sistemática. Med Intensiva. 2020;44:80–87.