To study the effect of setting positive end-expiratory pressure (PEEP) in an individualized manner (based on highest static compliance) compared to setting PEEP according to FiO2 upon mortality at 28 and 90 days, in patients with different severity acute respiratory distress syndrome (ARDS).
SettingA Spanish medical–surgical ICU.
DesignA post hoc analysis of a randomized controlled pilot study.
PatientsPatients with ARDS.
InterventionsVentilation with low tidal volumes and pressure limitation at 30cmH2O, randomized in two groups according to the method used to set PEEP: FiO2-guided PEEP group according to FiO2 applied and compliance-guided group according to the highest compliance.
Primary variables of interestDemographic data, risk factors and severity of ARDS, APACHE II and SOFA scores, daily Lung Injury Score, ventilatory measurements, ICU and hospital stay, organ failure and mortality at day 28 and 90 after inclusion.
ResultsA total of 159 patients with ARDS were evaluated, but just 70 patients were included. Severe ARDS patients showed more organ dysfunction-free days at 28 days (12.83±10.70 versus 3.09±7.23; p=0.04) and at 90 days (6.73±22.31 vs. 54.17±42.14, p=0.03), and a trend toward lower 90-days mortality (33.3% vs. 90.9%, p=0.02), when PEEP was applied according to the best static compliance. Patients with moderate ARDS did not show these effects.
ConclusionsIn patients with severe ARDS, individualized PEEP selection based on the best static compliance was associated to lower mortality at 90 days, with an increase in organ dysfunction-free days at 28 and 90 days.
Estudiar el efecto de programar la presión positiva al final de la espiración (PEEP) de manera individualizada (basada en la mejor complianza estática) comparada con la programada según la FiO2 sobre la mortalidad a 28 y 90 días, en pacientes con diferente gravedad de síndrome de distrés respiratorio agudo (SDRA).
ÁmbitoUCI española médico-quirúrgica.
DiseñoAnálisis post hoc de un estudio piloto controlado y aleatorizado.
PacientesPacientes con SDRA.
IntervencionesVentilación con volúmenes tidales bajos y presión limitada a 30cmH2O, divididos en función de la manera de programar la PEEP: según la fracción inspirada de oxígeno o la mejor complianza estática pulmonar.
Variables de interés principalesDatos demográficos, factores de riesgo y gravedad del SDRA, escalas APACHE II y SOFA, Escala de Daño Pulmonar diaria, parámetros ventilatorios, estancia en UCI y hospitalaria, fracaso orgánico y mortalidad a día 28 y 90.
ResultadosValoramos 159 pacientes con SDRA, de los que se incluyeron 70. En los pacientes con SDRA grave, observamos un mayor número de días sin fracaso multiorgánico a los 28 (12,83±10,70 vs. 3,09±7,23, p=0,04) y 90 días (6,73±22,31 vs. 54,17±42,14, p=0,03), y una menor mortalidad a 90 días (33,3% vs. 72,7%, p=0,16), cuando la PEEP se programaba según la mejor complianza estática. No encontramos dichos efectos en el SDRA moderado.
ConclusionesEn pacientes con SDRA grave, programar la PEEP según la mejor complianza estática se asocia a una menor mortalidad a 90 días y a un aumento de los días libres de fracaso multiorgánico a 28 y 90 días.
Although mechanical ventilation is potentially lifesaving in patients with acute respiratory distress syndrome (ARDS), it may cause ventilator-associated lung injury.1 In 2000 the ARDSNetwork study demonstrated that ventilation with low tidal volume (VT) and positive end expiratory pressure (PEEP) application may reduce ARDS mortality near 9%.2 So, protective mechanical ventilation with lower tidal volume (VT) and PEEP application was generalized. Nonetheless, the mortality of ARDS still remains high.3,4
Ventilation with PEEP is essential for patients with ARDS, as it was early observed that PEEP greatly improves oxygenation in ARDS,5 but also it may induce lung injury. High PEEP levels may open collapsed alveoli and decrease intrapulmonary shunt and avoid repetitive alveolar opening and closing during the respiratory cycle, but also may induce overdistension promoting lung injury.6,7 Several methods have been proposed to set the level of PEEP, although the preferred method is still controversial.7,8
Previously, we conducted an open, randomized controlled pilot study9 to test the hypothesis that individualized PEEP set based on highest compliance would improve oxygenation, compared to setting PEEP based on FiO2 (fraction of inspired oxygen) according to ARDSNetwork study.2 We found that, although there were not differences on oxygenation, patients with ARDS ventilated with PEEP determined according their highest compliance had a strong trend toward lower mortality at day 28 and a significant increase in organ-dysfunction-free days at 28 days.9
This current study is a post hoc analysis of our earlier study.9 We stratified patients with ARDS according to the last Berlin definition, evaluating the mortality at day 28 and at day 90 as primary end-points, and some important outcomes as secondary end-points (oxygenation, respiratory parameters, presence and length of multiple organ failure, length of mechanical ventilation) following the new Berlin definition of ARDS.9,10 We hypothesized that patients who received an individualized PEEP setting based on highest compliance would improve their outcomes according to severity of ARDS.
Patients and methodsAlthough previously reported, our original study which this current unplanned post hoc analysis is based on was an open, randomized controlled pilot study to test the hypothesis that individualized PEEP set based on highest compliance would improve oxygenation, compared to setting PEEP based on FiO2.9
It was conducted in a 14-bed medical-surgical intensive care unit (ICU) in Spain during 60 months (2002–2007), after being approved by our institution's Ethics and Clinical Trials Committee (protocol number: 18/2006) and registered on clinicaltrials.gov with the number: NCT01119872. Written informed consent was required for inclusion and obtained from the nearest relatives of the patient.
We included all patients with ARDS who meet criteria for ARDS according to the American-European Consensus Conference definition11 before and after 24h under mechanical ventilation, ventilated with low VT (6–8ml/kg predicted body weight (PBW)), an inspiratory plateau pressure below 30cmH2O, initial respiratory rate of 30breaths/min adjusted to maintain a pH between 7.30 and 7.45 and limited to a maximum of 35breaths/min, FiO2 ensuring arterial oxygen saturation (SaO2) 88–95% or arterial partial oxygen pressure (PaO2) of 55–80mmHg, and a PEEP level adjusted to achieve the best oxygenation with the lowest FiO2 without adverse hemodynamic effects. If the plateau pressure was greater than 30cmH2O with VT of 6ml/kg PBW, a stepwise reduction of VT of 1ml/kg PBW to 5 and 4ml/kg/PBW was allowed. If this was the case, the plateau pressure limit was set at 35cmH2O.
Patients were excluded if they declined enrolment (refusal of nearest relatives of patient to sign the informed consent), younger than 18 years, pregnant, or had neuromuscular disease, intracranial hypertension, head trauma, left ventricular dysfunction (on echocardiography), patients under mechanical ventilation more than 72h, had barotrauma or developed it during the first 24h of meeting ARDS inclusion criteria (defined as the presence of air outside the tracheobronchial tree, resulting from presumed alveolar rupture, and manifested as interstitial emphysema, pneumothorax, pneumomediastinum, pneumoperitoneum, or subcutaneous emphysema12), and patients with end-stage conditions (death expected within 90 days) were excluded.
After 24h of inclusion, patients were randomized into two groups according to PEEP determination method: FiO2-guided PEEP (control group) or compliance-guided PEEP. Randomization was performed in blocks of 10 using sealed envelopes. In the control group, PEEP was set based on the subject's FiO2, as applied in the ARDSNetwork study,2 while in the compliance-guided group, PEEP was set daily, according to the best compliance measured by the method described by Suter et al.13 Static compliance (Cst) was measured at increasing levels of PEEP. Cst was calculated dividing VT by the pressure difference at end of inflation hold (2s) and PEEP was increased at steps of 2 cmH2O beginning at 5cmH2O, without an upper PEEP titration limit. The highest Cst was considered to be the best PEEP. If at two different PEEP levels Cst was identical, we chose the one with the lower plateau pressure. Driving pressure was calculated as the plateau pressure minus PEEP.14
To set PEEP level, all subjects received sedatives and opioids at the time of PEEP setting (neuromuscular blocking agents only if they required it for low VT ventilation, although not for the measurement of intrinsic PEEP or plateau pressure).
Except for the way of setting PEEP, all other ventilator parameters and weaning protocol followed the same rules in both study groups. Weaning was begun if the cause of respiratory failure had resolved, PaO2 was higher than 60mmHg with a FiO2 at 0.4 or less and PEEP level below 6cmH2O. In patients in the compliance-guided group, PEEP level was lowered stepwise by 2cmH2O. In the control group the protocol described in the ARDSNet study was applied.2
Routine care (sedation, analgesia) or special requirements (hemodynamic support, antibiotics, etc.) other than mechanical ventilation were guided according to our local protocols followed by the attending physicians not involved in the study.
We collected data from each patient about sex, age, risk factors for ARDS, routine laboratory measurements, Acute Physiology and Chronic Health Evaluation II score (APACHE II),15 daily Lung Injury Score (LIS),16 Sepsis-Related Organ Failure Assessment score (SOFA),17 multiple organ dysfunction score,18 length of mechanical ventilation, length of ICU and hospital stay, mortality at day 28 and at day 90 after inclusion, pulmonary variables, physiologic measurements, ventilatory variables, cardiovascular variables, adverse events, extra-pulmonary organ failures, sedation, and daily chest X-ray. All variables and data were recorded at study inclusion, at 6h after inclusion, and between 6:00a.m. and 8:00a.m. on days 1, 2, 3, 4, 7, 14, 21, and 28.
In our present study, we retrospectively examined mortality at 28 and at 90 days in subgroups of patients with moderate or severe ARDS according to the last Berlin definition of ARDS10 (there were no patients with mild ARDS included in the study), defined by the worse oxygenation data (measured as PaO2/FiO2) at baseline and/or in the 24-h period immediately after randomization. The last Berlin definition divided the severity of ARDS into three groups according to the degree of hypoxemia (measured as PaO2/FiO2): (a) mild: 200mmHg<PaO2/FiO2≤300mmHg, (b) moderate: 100mmHg<PaO2/FiO2≤200mmHg, and (c) severe: PaO2/FiO2≤100mmHg.10 We also examined oxygenation improvement, ventilator-free days at day 28 and at day 90, ICU and hospital stay, multiple organ dysfunction (MOD) free days at 28 day and at day 90, respiratory and hemodynamic parameters. Organ failure is defined as a SOFA score17 greater than 2 and MOD requires 2 or more organ failures. Organ dysfunction-free days were defined as days alive and free of any organ dysfunction,19,20 and ventilator – free days as days of unassisted breathing, both calculated at 28 days (all deaths occurring prior to day 28 were considered as zero organ dysfunction-free or ventilator-free days).2
For the statistical analysis, we used statistics software (SPSS 15.0, SPSS, Chicago, IL). We first tested normality of data distribution with the Kolmogorov–Smirnov test. Variables are described as mean±SD in case of quantitative variables with normal distribution, medians and interquartile ranges in case of quantitative variables with non-normal distribution and percentages in case of qualitative variables. They were compared using the Student t test, the Mann–Whitney test and the chi-square test respectively. Kaplan–Meier analysis with log-rank test was applied to compare survival at 28 days between groups.
Statistical significance was set at p<0.05, and results are expressed with their 95% confidence intervals.
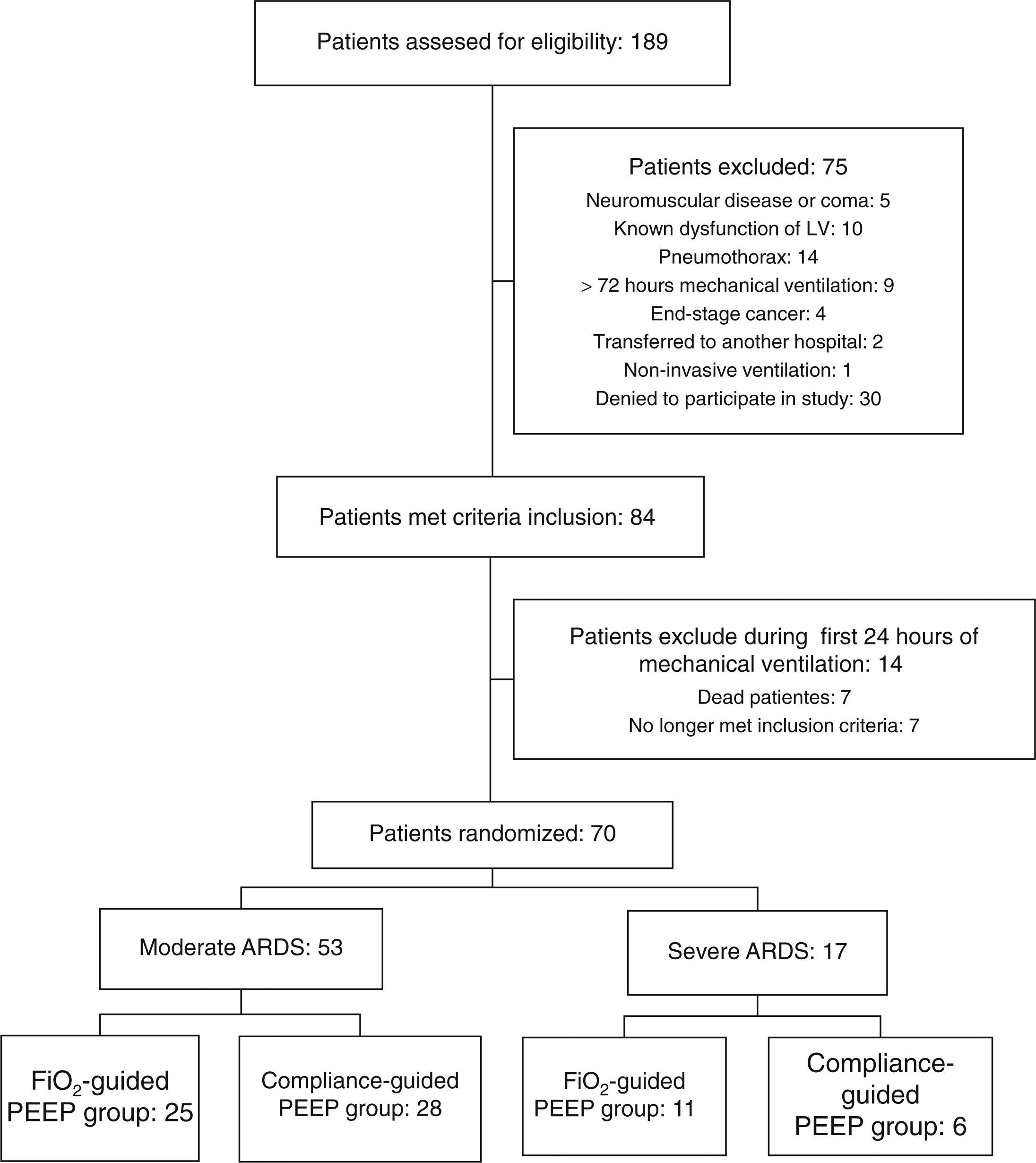
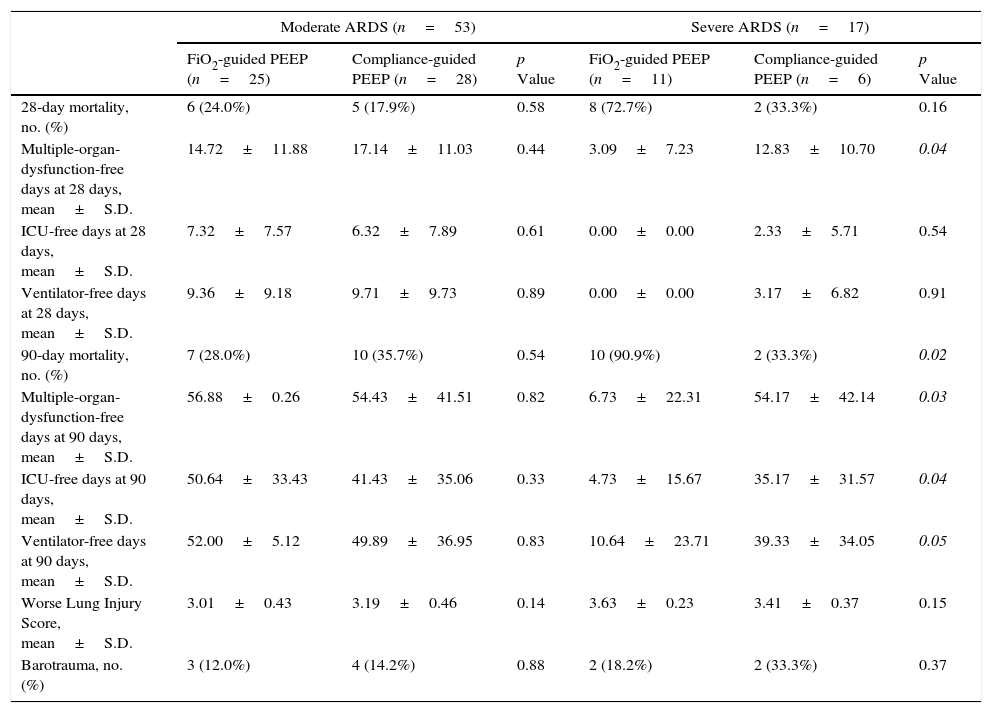
ResultsA total of 70 patients were included in the study: 53 patients with moderate ARDS and 17 with severe ARDS. Flow diagram of study is shown in Fig. 1.
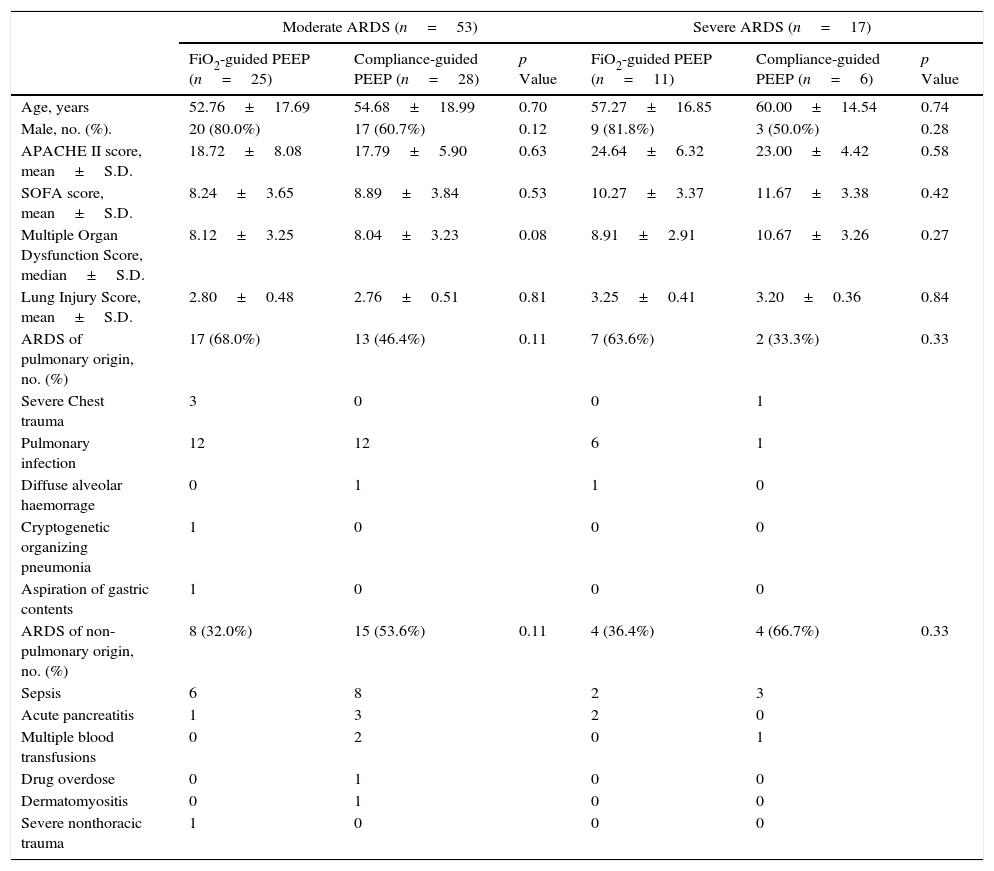
At baseline, there were not differences on age, sex, severity of ARDS, severity of illness and causes of ARDS between both groups of study in either severe or moderate ARDS (Table 1).
Baseline characteristics of subjects at study inclusion according to ARDS severity.
| Moderate ARDS (n=53) | Severe ARDS (n=17) | |||||
|---|---|---|---|---|---|---|
| FiO2-guided PEEP (n=25) | Compliance-guided PEEP (n=28) | p Value | FiO2-guided PEEP (n=11) | Compliance-guided PEEP (n=6) | p Value | |
| Age, years | 52.76±17.69 | 54.68±18.99 | 0.70 | 57.27±16.85 | 60.00±14.54 | 0.74 |
| Male, no. (%). | 20 (80.0%) | 17 (60.7%) | 0.12 | 9 (81.8%) | 3 (50.0%) | 0.28 |
| APACHE II score, mean±S.D. | 18.72±8.08 | 17.79±5.90 | 0.63 | 24.64±6.32 | 23.00±4.42 | 0.58 |
| SOFA score, mean±S.D. | 8.24±3.65 | 8.89±3.84 | 0.53 | 10.27±3.37 | 11.67±3.38 | 0.42 |
| Multiple Organ Dysfunction Score, median±S.D. | 8.12±3.25 | 8.04±3.23 | 0.08 | 8.91±2.91 | 10.67±3.26 | 0.27 |
| Lung Injury Score, mean±S.D. | 2.80±0.48 | 2.76±0.51 | 0.81 | 3.25±0.41 | 3.20±0.36 | 0.84 |
| ARDS of pulmonary origin, no. (%) | 17 (68.0%) | 13 (46.4%) | 0.11 | 7 (63.6%) | 2 (33.3%) | 0.33 |
| Severe Chest trauma | 3 | 0 | 0 | 1 | ||
| Pulmonary infection | 12 | 12 | 6 | 1 | ||
| Diffuse alveolar haemorrage | 0 | 1 | 1 | 0 | ||
| Cryptogenetic organizing pneumonia | 1 | 0 | 0 | 0 | ||
| Aspiration of gastric contents | 1 | 0 | 0 | 0 | ||
| ARDS of non-pulmonary origin, no. (%) | 8 (32.0%) | 15 (53.6%) | 0.11 | 4 (36.4%) | 4 (66.7%) | 0.33 |
| Sepsis | 6 | 8 | 2 | 3 | ||
| Acute pancreatitis | 1 | 3 | 2 | 0 | ||
| Multiple blood transfusions | 0 | 2 | 0 | 1 | ||
| Drug overdose | 0 | 1 | 0 | 0 | ||
| Dermatomyositis | 0 | 1 | 0 | 0 | ||
| Severe nonthoracic trauma | 1 | 0 | 0 | 0 | ||
No., number of patients; S.D., standard deviation; APACHE II, Acute Physiology and Chronic Health Evaluation II score; SOFA, sepsis-related organ failure assessment score.
Mortality at day 28 was higher in patients with severe ARDS (58.8%) than in those with moderate ARDS, (20.8%, p=0.003); and also at 90 days (70.6% on severe versus 32.1%, p=0.005). We found a trend toward lower mortality on day 28 in compliance-guided group in patients with severe ARDS (33.3% in compliance-guided group versus 72.7% in FiO2-guided PEEP group, p=0.16), with a lower mortality on day 90 in patients with severe ARDS (33.3% in compliance-guided group versus 90.9% in FiO2-guided PEEP group, p=0.02). There were no differences on 28-days nor 90-days mortality between both groups of treatment in patients with moderate ARDS (Table 2). Table 2 also shows other important clinical outcomes according to severity of ARDS.
Clinical outcomes according to ARDS severity.
| Moderate ARDS (n=53) | Severe ARDS (n=17) | |||||
|---|---|---|---|---|---|---|
| FiO2-guided PEEP (n=25) | Compliance-guided PEEP (n=28) | p Value | FiO2-guided PEEP (n=11) | Compliance-guided PEEP (n=6) | p Value | |
| 28-day mortality, no. (%) | 6 (24.0%) | 5 (17.9%) | 0.58 | 8 (72.7%) | 2 (33.3%) | 0.16 |
| Multiple-organ-dysfunction-free days at 28 days, mean±S.D. | 14.72±11.88 | 17.14±11.03 | 0.44 | 3.09±7.23 | 12.83±10.70 | 0.04 |
| ICU-free days at 28 days, mean±S.D. | 7.32±7.57 | 6.32±7.89 | 0.61 | 0.00±0.00 | 2.33±5.71 | 0.54 |
| Ventilator-free days at 28 days, mean±S.D. | 9.36±9.18 | 9.71±9.73 | 0.89 | 0.00±0.00 | 3.17±6.82 | 0.91 |
| 90-day mortality, no. (%) | 7 (28.0%) | 10 (35.7%) | 0.54 | 10 (90.9%) | 2 (33.3%) | 0.02 |
| Multiple-organ-dysfunction-free days at 90 days, mean±S.D. | 56.88±0.26 | 54.43±41.51 | 0.82 | 6.73±22.31 | 54.17±42.14 | 0.03 |
| ICU-free days at 90 days, mean±S.D. | 50.64±33.43 | 41.43±35.06 | 0.33 | 4.73±15.67 | 35.17±31.57 | 0.04 |
| Ventilator-free days at 90 days, mean±S.D. | 52.00±5.12 | 49.89±36.95 | 0.83 | 10.64±23.71 | 39.33±34.05 | 0.05 |
| Worse Lung Injury Score, mean±S.D. | 3.01±0.43 | 3.19±0.46 | 0.14 | 3.63±0.23 | 3.41±0.37 | 0.15 |
| Barotrauma, no. (%) | 3 (12.0%) | 4 (14.2%) | 0.88 | 2 (18.2%) | 2 (33.3%) | 0.37 |
No., number of patients; S.D., standard deviation; ICU, intensive care unit.
Italic values: p≤0.05.
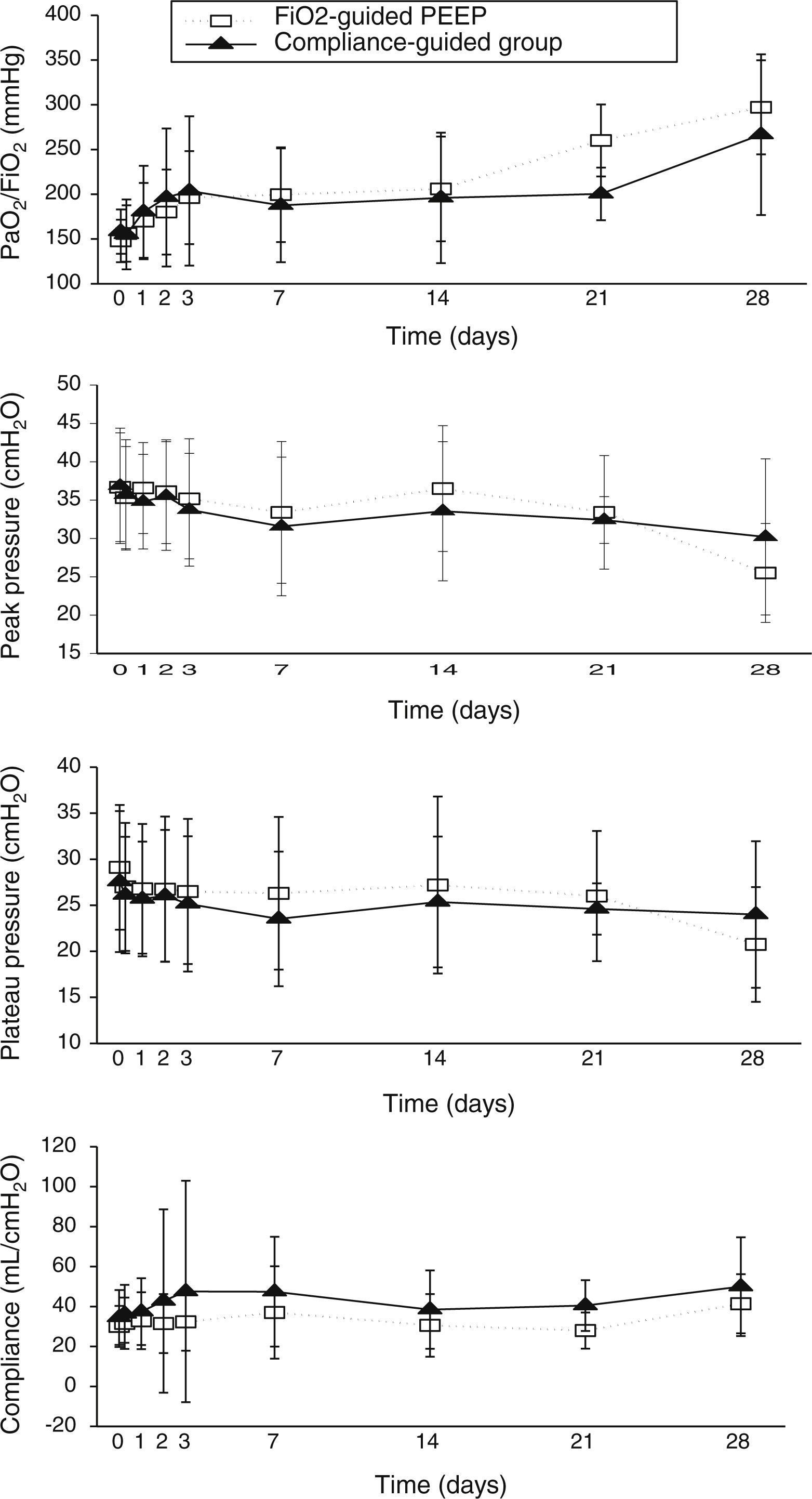
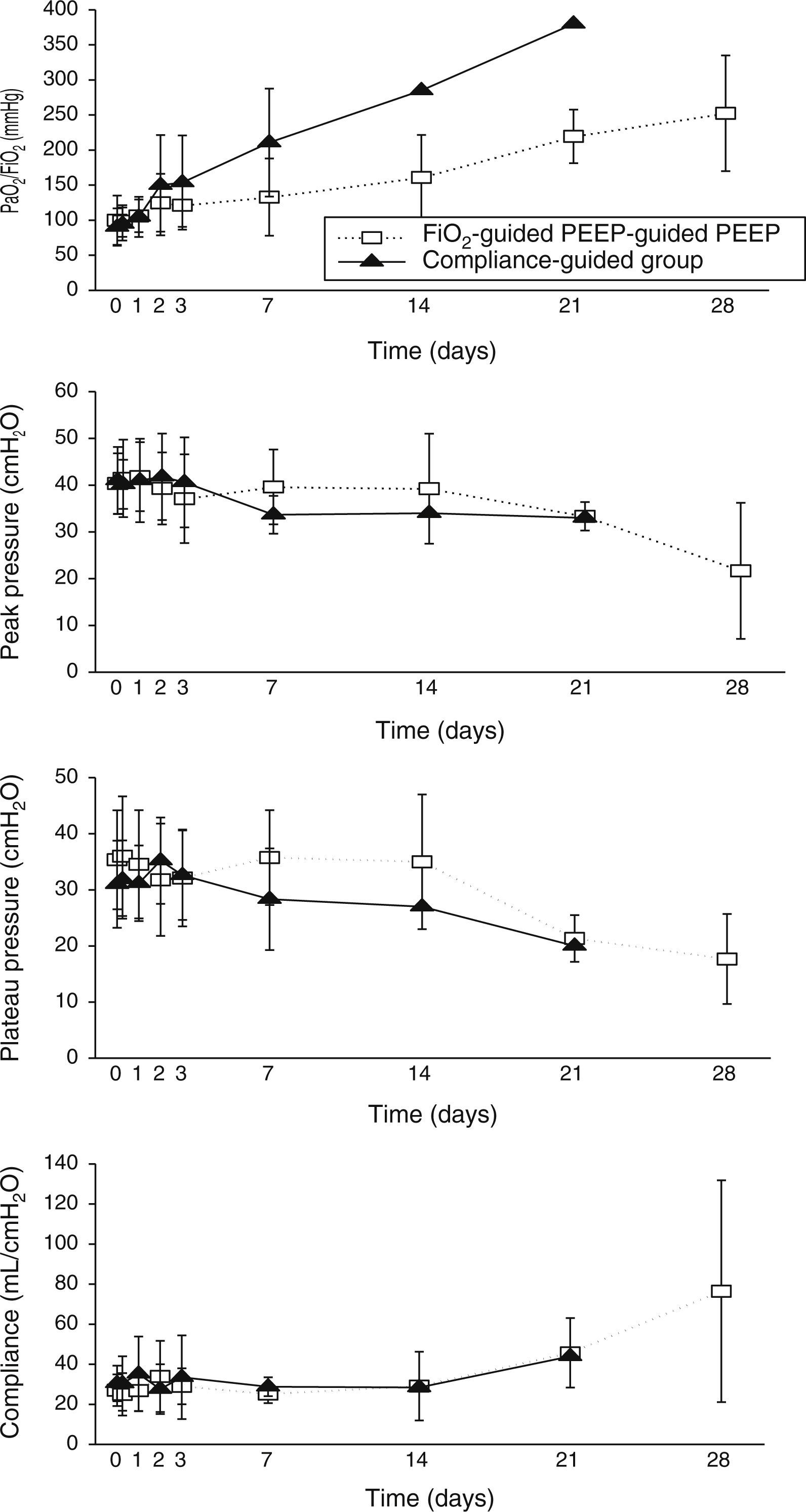
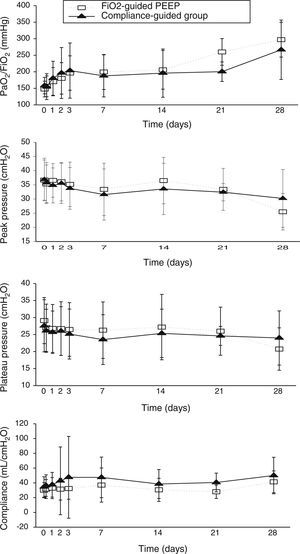
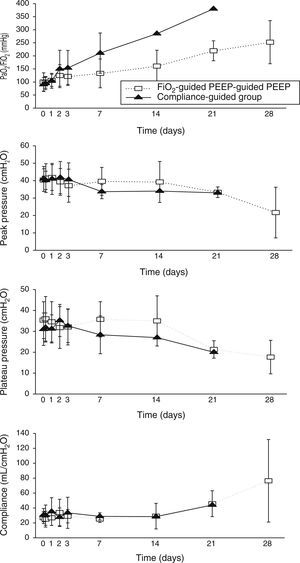
Concerning respiratory parameters, we found that there was no difference in mean PEEP at study entry between compliance-guided group and FiO2-guided PEEP group on moderate and severe ARDS (complete data of respiratory variables during the study are shown in supplementary material). Among the patients with moderate ARDS, there were no differences on compliance, peak and plateau pressures or oxygenation during the study (Fig. 2). However, among patients with severe ARDS, there was a trend toward better oxygenation in the compliance-guided group beyond first day of study, without differences in respiratory pressures (Fig. 3). It is important to remark a strong trend to lower driving pressure between patients included in compliance-guided PEEP adjustment group in both groups of severity of ARDS.
There were no differences in other respiratory parameters, the presence of auto-PEEP (we did not find any patient with auto-PEEP), pH and level of hypercapnia, physiologic measurements, cardiovascular measurements, adverse events and development of barotrauma between both groups of study.
DiscussionThis unplanned post hoc subgroups analysis found that protective mechanical ventilation with low VT, airway pressure limited to 30cmH2O and compliance-guided PEEP adjustment, compared to FiO2-guided PEEP in patients with severe ARDS, was associated with lower mortality at 90 days, and also, a shorter duration of multiple organ dysfunction at 28 and at 90 days and lower duration of mechanical ventilation at 90 days. However, these benefits are not achieved on moderate ARDS.
The new Berlin definition of ARDS shows that the more severe the ARDS is the higher the mortality and the length of mechanical ventilation are.10 We also found higher mortality rate between patients with severe ARDS. However, Lopez Saubidet et al. observed that there were no differences on hospital and 6 month mortality between patients with moderate or severe ARDS admitted to ICU.21 Some authors10,22 postulate that the new classification of ARDS may identify subgroups of patients who may benefit from specific adjunctive interventions, as prone position23 and maybe the way of setting PEEP. In that way, recently, Mansur et al. have reported a benefit on mortality in patients with severe ARDS with statin treatment, benefit that was not shown on mild or moderate ARDS.24
Unexpectedly, we found higher mortality at 90 days on severe ARDS than expected compared to the Berlin definition and to the level of severity of illness, being this different due to an increased mortality in patients with severe ARDS included on the FiO2-guided PEEP group. This might be due to the small number of patients with severe ARDS included in our study.
An individualized PEEP setting may improve outcomes and oxygenation on ARDS patients.9,20,25,26 None of these studies have demonstrated a decrease in mortality in the group treated with individualized PEEP. We find that PEEP selection based on best static compliance in patients with severe ARDS seems to improve oxygenation rolling along the evolution of disease. However, we did not find a significant difference in PEEP levels between the two groups of treatment. When we compared the PEEP level that the patients included on compliance-guided PEEP group should have received with the one they should have received according to the FiO2 applied, we found it was different (higher or lower PEEP levels than those prescribed according to the PEEP/FiO2 table) in 85% of patients with severe ARDS and 66% of patients with moderate ARDS. Thus, similar mean PEEP values in our opinion do not exclude that, individual compliance-guided settings may be distributed over a wider range of values and be associated with less ventilator-induced lung injury.
Recently, Amato et al.14 performed a multilevel mediation analysis with nine previous randomized trials on patients with ARDS2,20,25,27–32 to examine if the driving pressure (VT/respiratory-system compliance) was an independent variable associated with survival. They found that the increase of driving pressure was directly associated with an increment of mortality at 60 days, even in patients receiving “protective” plateau pressures (limited to 30cmH2O) and low VT. The survival benefits found in the VT trials2,27–30 were proportional to reductions in driving pressure driven by treatment-group assignment rather than to reductions in VT; similarly, the survival benefits observed in the PEEP trials20,25,31,32 occurred in relation to reductions in driving pressure. This study supports our theory that PEEP effect is beneficial when, applied individually, it improves compliance. Interestingly, we have found that patients randomized to compliance-guided PEEP adjustment group had a strong trend to lower driving pressure mainly at the beginning of the evolution of the disease. This finding was very similar regardless of severity of ARDS.
We also observed that customizing PEEP to the individual subject was associated with shorter duration of MOD at 28 days on severe ARDS, which correlates with a shorter duration of ICU stay and length of mechanical ventilation. These findings were not shown in the moderate ARDS group. Several studies2,33–35 have demonstrated that an array of inflammatory cytokines is released into the systemic circulation as a consequence of high VT or high PEEP, which correlates with higher morbidity and mortality, and cytokine release might be proportional to the severity of the ARDS. Unfortunately, we did not measure inflammatory cytokines to support the findings on MOD.
One of our strengths is that we only included patients who full-fillled the ARDS criteria11 after 24h of mechanical ventilation. Several studies have demonstrated that after a short period of mechanical ventilation, patients may improve their PaO2/FiO2 ratio above 200mmHg and this improvement may be associated with lower mortality.36–38 Villar et al.39 demonstrated that there are a marked PaO2/FiO2 variability within the first 24h of ARDS diagnosis that may introduce a bias in the assessment of ARDS severity. And to the best of our knowledge, this investigation is the first to evaluate the impact of individualized PEEP on the mortality of patients with ARDS according to the new Berlin definition of ARDS.10
Our study has several limitations. It is a retrospective analysis of a previous pilot study9 designed to determine if an individualized level of PEEP on patients with ARDS improves oxygenation, and carried out in a single center; so, we have a small sample size with a reduced number of patients on each group which limits the possibility of a multivariate analysis of mortality. The study was un-blinded and bias cannot be excluded. Throughout the study there were some difficulties in setting PEEP at best compliance: sometimes several time-consuming attempts were required to find the best PEEP in the compliance group, including the need for muscle relaxants, or the study procedures had to be interrupted to allow for endotracheal suctioning.
In conclusion, our study showed that individualized PEEP setting based on the best static compliance in subjects with ARDS treated with low VT and limited plateau pressure benefit patients with severe ARDS, according to new Berlin definition.10 The results showed a lower mortality at day 90, with a significant increase in organ-dysfunction-free days at 28 and 90 days and a trend toward a lower driving pressure, although oxygenation did not improve. No benefits were found on patients with moderate ARDS. It seems clear that to individualize PEEP level in patients with ARDS according to the severity, may improve the outcomes of these patients. Further studies are necessary to clearly address this question.
Financial disclosureThis study nor any of the authors have received some funding for carrying out the same.
Conflict of interestNo author has a conflict of interest regarding this study.
All patients and staff who have made this study possible.
Current address: Unidad de Cuidados Intensivos, Hospital Universitario Príncipe de Asturias, Carretera Alcalá-Meco SN, Alcalá de Henares, Madrid 28805, Spain.
Current address: Profesor asociado Medicina, Universidad de Alcalá, Alcalá de Henares, Madrid, Spain.
Current address: Unidad de Cuidados Intensivos, Hospital Universitario Ramón y Cajal, Carretera de Colmenar Viejo Km 9.100, Madrid 28034, Spain.
Current address: Unidad de Cuidados Intensivos, Hospital Universitario Fundación Jiménez Díaz, Avda Reyes Católicos 2, Madrid 28040, Spain.
- Home
- All contents
- Publish your article
- About the journal
- Metrics
- Read in English
- Download PDF
- Bibliography
- Additional material