Edited by: Alberto García-Salido - Pediatric Intensive Care Unit, Hospital Infantil Universitario Niño Jesús, Madrid, Spain
Last update: May 2024
More infoTo compare the safety and effectiveness of Continuous Positive Airway Pressure (CPAP) vs. High Flow Nasal Cannula (HFNC) to prevent therapeutic failure and the need of invasive ventilation in children with acute moderate-severe bronchiolitis.
DesignA systematic review and meta-analysis.
SettingMedline, Embase, Lilacs, Cochrane and gray literature (May 2020) was performed.
ParticipantsRandomized clinical trials patients with moderate to severe bronchiolitis.
Main variablesTherapeutic failure, need for invasive ventilation, adverse events, length of PCCU and of hospital stay.
InterventionThe quality of the studies was assessed with the Cochrane risk and bias tool. We conducted meta-analysis using fixed effect model and random effects model.
ResultsThree RCTs were included. Showed less risk of therapeutic failure with CPAP compared with HFNC (RR=0.7; 95%CI 0.5–0.99) developed hours later in patients with CPAP (MD=3.16; 95%CI 1.55–4.77). We did not find differences in other outcomes, such as need of invasive ventilation (RR=0.60; 95%CI 0.25–1.43), apnea (RR=0.40; 95%CI 0.08–1.99), or number of days in the intensive care unit (MD=0.02; 95%CI −0.38 to 0.42), and length of hospitalization (MD=−1.00; 95%IC −2.66 to 0.66). Adverse events (skin lesions) were more common with CPAP (RR 2.47; 95%CI 1.17–5.22).
ConclusionsIn moderate/severe bronchiolitis CPAP demonstrated a lower risk of therapeutic failure and a longer time to failure. But more adverse events like nasal injury. There were no differences in other variables.
Comparar la seguridad y la efectividad de la presión positiva continúa en la vía aérea (CPAP) y la cánula nasal de oxígeno de alto flujo (OAF) para prevenir el fracaso terapéutico y la necesidad de ventilación mecánica invasiva en niños con bronquiolitis aguda moderada y grave.
DiseñoRevisión sistemática y metaanálisis.
ÁmbitoBúsqueda en Medline, Embase, Lilacs, Cochrane y literatura gris (hasta mayo 2020).
ParticipantesEnsayos clínicos aleatorizados en pacientes con bronquiolitis aguda moderada-grave.
IntervencionesLa calidad de los estudios se evaluó utilizando la escala de riesgo de sesgos de Cochrane y se realizó un metaanálisis usando modelo de efectos fijos y de efectos aleatorios.
VariablesFracaso terapéutico, necesidad de ventilación invasiva, eventos adversos, estancia en la UCIP y en hospitalización.
ResultadosTres estudios fueron incluidos. Evidenciamos menor riesgo de fracaso terapéutico en los pacientes con CPAP comparados con CAF (RR: 0,7; IC 95%: 0,5-0,99), y este se desarrolló más tarde en los pacientes con CPAP (MD: 3,16; IC 95%: 1,55-4,77). No hubo diferencias en otras variables, como la necesidad de ventilación invasiva (RR: 0,60; IC 95%: 0,25-1,43), apnea (RR: 0,40; IC 95%: 0,08-1,99), estancia en la UCIP (MD: 0,02; IC 95%: −0,38-0,42) y en hospitalización (MD: −1,00; IC 95%: −2,66-0,66). Los eventos adversos (lesiones en piel) fueron más comunes en CPAP (RR: 2,47; IC 95%: 1,17-5,22).
ConclusionesEn bronquiolitis moderada y grave el CPAP demostró menor riesgo de fracaso terapéutico y una aparición más tardía, pero más eventos adversos (lesiones en piel). No encontramos diferencias en otras variables.
Acute bronchiolitis is still one of the most frequent diseases in children under two years, and it is also a common cause of hospitalization with considerable use of health resources.1,2 Despite the availability of multiple therapies, only supplementary oxygen and hydration have shown a beneficial impact on the evolution of these patients.1,2
Multiple microorganisms are associated with this disease, but respiratory syncytial virus (RSV) is by far the most common pathogen involved.3,4 The clinical manifestations range from a mild disease with a spontaneous resolution to a severe disease that could lead to respiratory failure and the need for ventilatory support. Infants and children who develop a severe disease require hospitalization in the pediatric critical care unit (PCCU) for monitoring and supplementary oxygen delivery according to its severity.
In the last decade, the use of non-invasive respiratory therapies such as the Nasal Continuous Positive Air Pressure (CPAP), High-Flow Nasal Cannula (HFNC) and the Non-invasive Ventilation (NIV)5,6 have gained popularity in the pediatric intensive care field. All these therapies appear as therapeutic alternatives to the orotracheal intubation and conventional invasive ventilatory support, to minimize its associated risks such as barotrauma associated to the use of positive airway pressure, healthcare-associated infections, airway injury related to intubation, thoracic air leakage, longer length-of-stay, deconditioning associated to sedatives and neuromuscular blockers and subglottic stenosis, among others.7,8
A number of clinical trials have evaluated the efficacy of HFNC and CPAP. Most of these trials and the available systematic reviews that have summarized this evidence have compared each intervention to standard care (i.e., oxygen therapy).9,10 The evidence has shown that both interventions are associated with a significant reduction in the incidence of treatment failure and improvement in some physiological variables. As of to date, there are two recent systematic reviews that have evaluated the effect of CPAP vs. HFNC. One of them conducted by Moorel et al.11 did not conduct meta-analysis. Authors concluded that treatment failure seemed to be higher in children from the HFNC group compared to the CPAP group, but there were no differences in the need for intubation, invasive ventilation, length of stay in PCCU service and length of oxygen therapy during the interventions.
In the recent review by Lin et al.,12 the authors conducted a meta-analysis of the available evidence of HFNC and CPAP in children with bronchiolitis. The authors evaluated the effectiveness of both interventions compared against control, and against each other and found that both, HFNC and CPAP were superior to control groups. When analyzing both interventions against each other, they found a significant increase in treatment failure events in the HFNC group compared with the CPAP group, without differences in other variables as hospital length of stay, length of time of oxygen supplementation (LOO), rate of transfer to intensive care unit, or incidence of intubation.
However, in this review, the authors included patients with any type of bronchiolitis (including children with mild bronchiolitis treated at the emergency department) regardless of their severity, which makes the result not entirely applicable to pediatric critical care units (PCCU) settings. Moreover, the authors did not perform subgroup analysis to determine the differences in the effect of these interventions in children with moderate to severe bronchiolitis who are already in a PCCU. Furthermore, the review by Lin et al. did not assess the quality of the body of evidence (also called the certainty of the evidence) to establish confidence in pooled estimates of the interventions (i.e., with the Grading of Recommendations, Assessment, Development, and Evaluation -GRADE methodology).13 As a result, there is no appropriate evidence synthesis to inform decisions in PCCU, and intensivists often have to face the uncertainty of which intervention might work better in children with a moderate to severe disease.
Given the popularity of these interventions but the lack of a structured evidence synthesis about the relative differences between these two alternatives, we hypothesized that CPAP leads to fewer therapeutic failures and fewer mechanical ventilation rates than HFNC. Thus, to reject a null hypothesis and accept our hypothesis, in this study we aimed to determine the comparative effectiveness between HFNC and CPAP in children with moderate to severe bronchiolitis in decreasing the need for invasive ventilation and therapeutic failure through a systematic review of the literature and meta-analysis.
MethodsWe performed a systematic review and meta-analysis following the guidelines of the PRISMA statement (Preferred Reporting Items for Systematic Reviews and Meta-Analyses).14 Our review was previously registered in the PROSPERO register of systematic reviews (CRD42018099616).
Eligibility criteriaWe included randomized clinical trials (RCTs) that included children<2 years old, with acute moderate or severe bronchiolitis. We considered studies that compared HFNC with CPAP. The primary outcomes were therapeutic failure (as defined by the studies) (Table 1 Supplemental Digital Content) and the need for invasive ventilation. The secondary outcomes were mortality, length of stay in the PCCU, length of hospitalization, the change in physiologic variables (heart and respiratory rate, oxygen saturation, PCO2) and adverse events. We excluded, and provided reasons for doing so, the articles that did not meet all the previous criteria about population, intervention, comparison, and at least one outcome of interest.
Database searchWe searched MEDLINE, EMBASE, LILACS, and COCHRANE Central, and gray literature in clinical trials databases (www.clinicaltrials.gov), from inception to May 2020. We performed manual searches of relevant articles referenced in the eligible studies. There were not language limits. The search strategy is detailed in the Supplemental Digital Content 1.
Studies selectionTwo reviewers (MLC, JCJ) screened the titles and abstracts independently and in duplicate. References considered eligible by at least one of the reviewers were obtained in full text. Two researchers (MLC, JCJ) reviewed full texts independently and in duplicate to determine their inclusion. Disagreements were resolved by consensus or by a third researcher (IDF).
Data extractionTwo reviewers independently and in duplicate (MLC, JCJ) extracted the data in a pre-established format. Information extracted included: General information (name of the author, year of publication, study design, title), population characteristics (bronchiolitis classification, age, RSV identification, history of prematurity or bronchopulmonary dysplasia), interventions and its comparator characteristics (type of HFNC, liters per minute delivery, CPAP positive pressure used, the fraction of inspiratory oxygen, and length of the intervention), and the outcomes’ data (number events, number of patients per group, or mean and standard deviations).
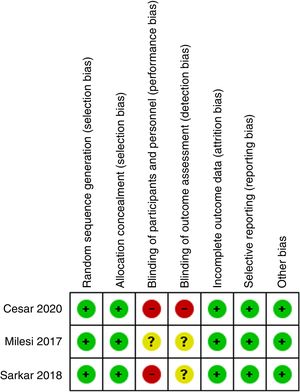
Risk of bias assessmentTwo reviewers (MLC, IDF) assessed the risk of bias (RoB) of the included studies with the Cochrane RoB tool.15 This tool considers the following criteria: random sequence generation, allocation concealment, blinding of patients and health providers, incomplete data, selective outcome reporting, and other potential sources of biases. Disagreements were resolved by consensus. The risk of publication bias among the studies was planned to be assessed by visual inspection of the funnel plot figure if we obtained more than 10 studies.16,17
Data synthesis and statistical methodsFor dichotomous outcomes, we calculated the relative risk (RR) and for continuous outcomes the mean difference, with their 95% confidence interval (95%CI). We conducted meta-analyses with the random-effects model for each outcome, if the heterogeneity was considered moderate to high, otherwise we used a fixed-effect model. Heterogeneity was assessed with the Chi-square test (Cochran's Q) considering a statistical significance when p values were <0.10. To determine the percent of total heterogeneity we used the I2 statistic calculated from Cochran's Q test result. We considered low heterogeneity when I2 was <25%, moderate if the value ranged from 25 to 50% and high if >50%.18A priori, we proposed subgroup analyses based on the severity of the disease (moderate vs severe) and on the etiology of the disease (RSV vs no RSV). We conducted the meta-analysis in the RevMan Software (Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014).
Quality of evidence assessmentTo assess the quality of the evidence (also called certainty of the evidence), we employed the GRADE approach which was applied by two reviewers independently and in duplicate. Disagreements were resolved by consensus. GRADE classifies the evidence as high, moderate, low, or very low quality based on considerations of risk of bias, inconsistency, indirectness, imprecision, and publication bias.13,19 We used the GRADE profiler (GRADEpro GDT; https://gradepro.org/) to generate the GRADE Summary of Findings table.
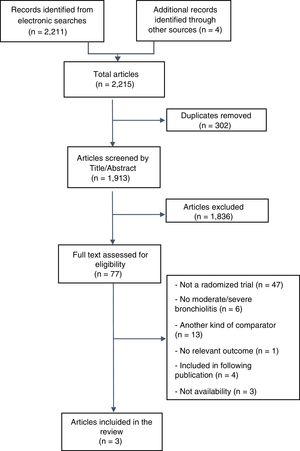
ResultsOur searches identified 2.215 titles and abstracts, of which 77 proved potentially eligible for full text evaluation, and 74 were excluded (see Fig. 1). The reasons for exclusion were as follows: (i) not randomized studies (N=47); (ii) were not performed exclusively in patients with moderate-to-severe bronchiolitis (N=6); (iii) had a different comparator (compared against standard oxygen therapy or comparison of different CPAP devices among them (N=13); (iv) they did not include at least one of the outcomes of interest (N=1); (v) were duplicates or included in subsequent publications (N=4); and (vi) no availability (it was not possible to contact the authors in order to check eligibility or to obtain information) (N=3) (see Fig. 1). The full list of excluded studies and the reasons are detailed in the Supplemental Digital Content 2.
Description of included studiesWe included three studies,20–22 which enrolled 236 infants. The mean age of children was 1.99 months (SD 1.59). Two studies reported the proportion of children with RSV.20,21 In these studies, RSV was identified in 179 patients (88%). Only one study21 included patients with a history of prematurity and bronchopulmonary dysplasia. The diagnosis of moderate to severe bronchiolitis was based on the following scales: Wood Downes, modified Wood score and/or Distress Assessment Index (RDAI).20–22Table 1 Supplemental Digital Content shows displays the characteristic of the included studies.
Given the nature of the interventions, it was not possible the blinding of patients/parents and health care providers to the allocated arm. Therefore, all the studies were judged as with high or unclear risk of detection and performance biases. The studies did not have significant losses of follow-up, and we did not identify additional sources of bias. Fig. 2 summarizes the risk of bias assessment. Lastly, the funnel plot for the main outcomes is presented in the Supplementary Digital Content Figs. 1 and 2. Unfortunately, the number of studies was very low and therefore, the interpretation of its results is limited, and we cannot determine with enough confidence the risk of publication bias.
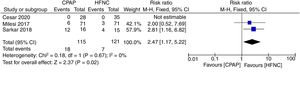
Primary outcomesThe meta-analysis showed a lower risk of therapeutic failure in the patients with CPAP in comparison to HFNC (RR 0.70; 95%CI 0.50–0.99; I2=0%, quality of evidence: low) (Fig. 3). The pooled estimate for invasive ventilation showed no differences between CPAP and HFNC (RR 0.60; 95%CI 0.25–1.43; I2=0%, quality of evidence: low) (Fig. 4). Fig. 5 Supplementary Digital Content presents the quality of the evidence for each outcome.
Therapeutic failure. Forest plot shows the relative risk (RR) for therapeutic failure. Horizontal bars denote 95% confidence intervals (95%CIs). Studies are represented as blue squares centered on the point estimate of the result of each study. The area of the square represents the weight given to the study in the meta-analysis. The black diamond represents the overall combined estimated effect and its 95%CI.
Invasive mechanical ventilation Forest plot shows the relative risk (RR) for the need for invasive mechanical ventilation. Horizontal bars denote 95% confidence intervals (95%CI). Studies are represented as blue squares centered on the point estimate of the result of each study. The area of the square represents the weight given to the study in the meta-analysis. The black diamond represents the overall combined estimated effect and its 95%CI.
Two studies examined the presence of apneas and found no differences between both therapies (RR 0.40; 95%CI 0.08–1.99, quality of evidence: moderate) (Fig. 5). Similarly, two studies examined the differences in the length of time until the development of therapeutic failure. Results show that the time to failure (in hours) was slightly higher in patients with HFNC compared to CPAP (mean difference, MD: 3.16; 95%CI 1.55–4.77, I2=0%, quality of evidence: moderate) (Fig. 6).
Apnea. Forest plot shows the relative risk (RR) for the presence of apnea. Horizontal bars denote 95% confidence intervals (95%CI). Studies are represented as blue squares centered on the point estimate of the result of each study. The area of the square represents the weight given to the study in the meta-analysis. The black diamond represents the overall combined estimated effect and its 95%CI.
Time until therapeutic failure. Forest plot shows the mean difference (MD) for the time until therapeutic failure, in hours. Horizontal bars denote 95% confidence intervals (95%CI). Studies are represented as blue squares centered on the point estimate of the result of each study. The area of the square represents the weight given to the study in the meta-analysis. The black diamond represents the overall combined estimated effect and its 95%CI.
We did not find differences in the length of time of therapy between both interventions (RR −0.19; 95%CI −0.42 to 0.04, I2=63%, quality of evidence: very low) (Fig. 3 Supplemental Digital Content), total length of time of non-invasive ventilatory support (MD: −1.07; 95%CI −2.14 to 0.0, quality of evidence: moderate) (Fig. 4 Supplemental Digital Content), length of stay in the PCCU (MD 0.02; 95%CI −0.38 to 0.42; I2=0%, quality of evidence: low) (Fig. 7), or in total length of stay in hospital (MD 0.00; 95%CI −0.57 to 0.57, quality of evidence: low)(Fig. 8). Regarding adverse events, we found a higher risk of having skin lesions in the CPAP group compared to the HFNC group (RR 2.47; 95%CI 1.17 to 5.22, quality of evidence: moderate (Fig. 9). None of the studies reported air leakage events.
Length of PCCU: Forest plot shows the mean difference (MD) for the length of stay in the PCCU, in days. Horizontal bars denote 95% confidence intervals (95%CI). Studies are represented as blue squares centered on the point estimate of the result of each study. The area of the square represents the weight given to the study in the meta-analysis. The black diamond represents the overall combined estimated effect and its 95%CI.
Length of stay in hospital. Forest plot shows the mean difference (MD) for the length of hospital stay, in days. Horizontal bars denote 95% confidence intervals (95%CI). Studies are represented as blue squares centered on the point estimate of the result of each study. The area of the square represents the weight given to the study in the meta-analysis. The black diamond represents the overall combined estimated effect and its 95%CI.
Adverse events. Forest plot shows the relative risk (RR) for adverse events. Horizontal bars denote 95% confidence intervals (95%CI). Studies are represented as blue squares centered on the point estimate of the result of each study. The area of the square represents the weight given to the study in the meta-analysis. The black diamond represents the overall combined estimated effect and its 95%CI.
In regard to the physiological variables, none of the studies reported the outcome as change (the difference between baseline and final post-treatment) in respiratory rate, heart rate, or SatO2. One of the trials measured the proportion of children that had an increase in the respiratory rate21 and the other study only measured the mean respiratory rate at specific points in time.22 These results were not suitable for the planned analyses in our protocol.
DiscussionIn this systematic review, we synthesized the evidence from three studies that analyzed 236 patients. We found that in patients with acute moderate to severe bronchiolitis, CPAP was superior to HFNC in terms of less therapeutic failures (an absolute reduction of 124 fewer failures per 1000 patients) and at a later moment in time (3.16h), without differences in the need for invasive ventilation. The quality of the evidence for these outcomes was considered as low due to the high risk of bias in the included trials and due to imprecision in the effect estimates “…due to imprecision in the effect estimates (GRADE quality assessments provided in the Supplemental Digital content).” Additionally, we did not find any difference between the interventions in terms of the effectiveness secondary outcomes (apneas, length of time of the use of the therapies evaluated, or in the PCCU length of stay or total length of stay in hospital). However, CPAP resulted in more adverse events related to nasal and skin lesions, with moderate quality of evidence.
Considering the physiopathology of the respiratory failure in the bronchiolitis, either CPAP or HFNC are useful interventions to improve the respiratory work of the patients with moderate or severe lung disease (with the subsequent decrease of invasive ventilatory support and its complications).23–25 The proposed mechanism of action of CPAP is based on the idea that positive end-expiratory pressure (PEEP) increases the residual functional capacity and the lung volume (avoiding alveolar collapse), and also increases the diameter of the airways (decreasing the resistance of the lower airways and avoiding the obstructive apnea), which is beneficial in patients with bronchiolitis.23
Observational studies had previously evaluated some physiologic outcomes after the beginning of CPAP in patients with bronchiolitis showing improvement after a few hours in some physiological variables: respiratory rate, heart rate, and partial pressure of CO2.23,26 Fleming et al. described how the introduction of CPAP was associated with reduced intubation and invasive ventilation rates, with no adverse events.27 Additional prospective studies comparing CPAP to conventional oxygen therapy in acute severe bronchiolitis showed a decrease in the inspiratory work with CPAP.28 An RCT showed a significant reduction in PCO2 with the use of CPAP compared to standard care29 and improvement in severity scores and the respiratory rate of the patients with CPAP compared to standard therapy.30,31
In the case of the HFNC, the heating and humidification of the gas at a high flow improves the minute volume of ventilation and decreases the dead space in the nasopharynx (decreasing the ventilatory work), improving mobilization of secretions and avoiding bronchial obstruction and inflammation due to the dry and cold air.32 Likewise, it is suggested that HFNC provides an unquantifiable degree of PEEP, which is lower in comparison to CPAP.
In a prospective observational study of the use of HFNC in the PCCU, in comparison to conventional oxygen therapy, physiologic changes in infants with bronchiolitis were reported.33 The authors described that the mean respiratory rate of infants with bronchiolitis decreased and that there were reductions in esophageal pressures and diaphragmatic activity as an estimate of the work of breathing in patients with bronchiolitis. Two large RCTs4,34 showed that the use of HFNC as initial therapy in patients with acute bronchiolitis who require oxygen has proven superior to conventional oxygen therapy. The most relevant outcome was the therapeutic failure, which was in favor of HFNC, and there were no differences in the need for orotracheal intubation, the time length of oxygen therapy and the length of stay in the PCCU and hospitalization. Therefore, the evidence has supported that both CPAP and HFNC work better than conventional care in bronchiolitis. For the first time, our study synthesizes the comparative evidence between the interventions in moderate to severe bronchiolitis in the PCCU.
Despite showing lower rates of therapeutic failures with CPAP compared to HFNC, we did not find differences in the need for invasive ventilatory support. As mentioned above, one potential explanation might be the decreasing use of orotracheal intubation in children with acute bronchiolitis thanks to the routine use of non-invasive ventilatory support. Both alternatives had reduced the risk for intubation, and therefore, the number of cases that will require it is much fewer than when only oxygen therapy is used.7
Despite we found no statistical differences in most of the effectiveness outcomes, except in therapeutic failure there was a trend in favor of CPAP over HFNC. The lack of statistical significance might be related to our analyses’ low statistical power due to the low number of events. Future studies with more patients will allow further analyses with higher sample size and will be helpful to determine if this trend will lead or not to statistically significant differences in those outcomes.
Although superior to HFNC, CPAP produced more adverse events, such as nasal and skin lesions. Previous reference studies that have studied the prevalence of nasal injury with the use of CPAP have been conducted in preterm newborns. The reported incidence of nasal injury has been as high as 100%, of which 80% were mild injuries (the most common was skin hyperemia), and none of them causing permanent sequels.35,36 Factors related to a higher risk of injury are more than 48h of use, inappropriate prongs sizes, inappropriate fixation techniques, and the lack of use of nasal barrier dressings.37 None of the included studies analyzed the previously described factors, and they also failed to report the severity of the injuries. Pediatric intensivists should always consider a trade-off between the beneficial effects and the harm produced by interventions. Considering that these adverse events are not severe, its potential occurrence might not be significant enough to prevent the use of an intervention that produces a relative reduction of the risk of failure, in approximately 30%. Moreover, we believe that controlling the mentioned risk factors for CPAP-related injuries may reduce their incidence. Other adverse events, such as air leaks, were not reported.21
Our study has several strengths to remark. We performed a comprehensive and systematic search of the literature in the main databases, we did not limit language or publication status, and we included gray literature searches, including clinical trials’ registries and conference’ proceedings. Also, we followed the highest methodological standards for the development of a systematic review of the literature according to Cochrane,14 and we evaluated the quality of evidence with the GRADE approach, which allows readers to interpret the evidence in light of the certainty around the results.
However, our review is not free of limitations. The low number of studies and patients restrained the development of additional analysis that we planned to conduct, such as subgroup or sensitivity analyses or the publication bias evaluation. The quality of evidence in several outcomes, including the primary ones, was judged as low quality, which was related to the risk of bias, and lack of precision related to the low number of patients and events of interest. However, we judged the quality of evidence for apnea and adverse events as moderate. Which means we are more confident that their results are closer to the truth for these outcomes.
In conclusion, we found, with low certainty, that CPAP is superior to HFNC in decreasing the probability of therapeutic failure (RR 0.70; CI 0.50–0.99; absolute risk reduction=12.4%), and when this event occurs, it appears later, in patients with CPAP compared with HFNC (more than 3h later). With moderate certainty, we found that CPAP produces more adverse events than HFNC, but these were local and non-severe. Future clinical trials with a more significant number of patients may confirm our results as more evidence might bring more precision to the results and, thus, a higher quality of the evidence. Therefore, more evidence will be crucial to confirm whether CPAP should be preferred over HFNC in patients with acute moderate-severe bronchiolitis admitted to the PCCU.
Authors’ contributionMLC: Participated in the conception and design of the study, the acquisition of data, the analysis and interpretation of data, the drafting of the article, and in the approval of the version to be submitted.
JCJ: Participated in the acquisition of data, the interpretation of data, revising the article critically for important intellectual content, and in the approval of the version to be submitted.
IDF: Participated in the conception and design of the study, the analysis and interpretation of data, the drafting of the article, and in the approval of the version to be submitted.
FundingNone.
Conflicts of interestNone of the authors declare any conflict of interest.