Intermittent glycemic measurements in patients admitted to the intensive care unit (ICU) can result in episodes of severe hypoglycemia or in a poor control of glycemia range. We designed a study to assess accuracy and reliability of continuous monitoring of tissue glucose for patients with distributive shock.
MethodsConsecutive patients admitted to the ICU with a diagnosis of distributive shock and the need of insulin infusion for glycemic control were included in the study. These patients were implanted a Continuous Glucose Control Monitoring System (CGMS) with the sensor inserted subcutaneously into the abdominal wall. CGMS values were recorded every 5min. Capillary glucose (CG) was monitored for adjusting insulin perfusion according to the ICU protocol. Correlation between both methods was assessed.
ResultsA total of 11,673 CGMS and 348 CG values were recorded. In five patients, CGMS failed to detect tissue glucose. A glucose value <3.33mmol/l (<60mg/dl) was observed in 3.6% of CGMS and in 0.29% CG values. 295 pairs of measurements were included in the statistical analysis for correlation assessment. The intraclass correlation coefficient was 0.706. The Pearson correlation coefficient was 0.71 (p<0.0001, 95% CI 0.65–0.76). The mean of differences between both measurement methods was 0.22mmol/l (3.98mg/dl) (95% CI 0.66–7.31).
ConclusionsWhen the Continuous Glucose Control Monitoring System (CGMS) is able to obtain data (75% of the patients), there is correlation between the values obtained by this method and capillary blood glucose in patients with distributive shock. CGMS can detect more episodes of glycemic excursions outside the normal range than intermittent capillary glucose monitoring. Variables that may impair glucose metabolism and peripheral soft tissues perfusion could impair CGMS measurements.
las medición de glucemia intermitente pueden provocar episodios de hipoglucemia severa o un mal control glucemico en los pacientes ingresados en la Unidad de Cuidados Intensivos (UCI). Diseñamos un estudio para evaluar la exactitud y fiabilidad de la monitorización continua de glucosa tisular en pacientes con shock distributivo.
MétodosSe incluyeron en el estudio todos los pacientes ingresados consecutivamente en la UCI con el diagnóstico de shock distributivo y la necesidad de insulina en perfusión para el control glucémico. A estos pacientes se les implantó un Sistema de Monitorización Continua de la Glucosa Tisular (CGMS) con un sensor insertado en tejido subcutáneo de la pared abdominal. CGMS valores se registraron cada cinco minutos. La glucosa capilar (GC) fue monitorizada para ajustar la perfusión de insulina de acuerdo con el protocolo de la UCI. Se evaluó la correlación entre ambos métodos.
ResultadosSe registraron un total de 11.673 valores de CGMS y 348 valores de CG. En cinco pacientes, la CGMS no pudo ser detectada. Un valor de glucosa <3,33mmol/l (<60mg/dl) se observó en 3,6% de los valores de CGMS y en el 0,29% de los valores de CG. 295 pares de mediciones se incluyeron en el análisis estadístico para la evaluación de la correlación. El coeficiente de correlación intraclase fue de 0,706. El coeficiente de correlación de Pearson fue de 0,71 (p<0,0001; IC 95% 0,65–0,76). La media de las diferencias entre los dos métodos de medición fue de 0,22mmol/l (3,98mg/dl) (IC 95% 0,66 a 7,31).
ConclusionesCuando el sensor de medición de glucosa tisular continua es capaz de obtener datos (75% de los pacientes), existe correlación entre los valores obtenidos mediante este método y la glucemia capilar en los pacientes que presentan shock distributivo. CGMS puede detectar más episodios de excursiones glucémicas fuera del rango de normalidad que la monitorización intermitente de glucosa capilar. Variables que pueden perjudicar el metabolismo de la glucosa y la perfusión periférica de los tejidos blandos podrían afectar las mediciones CGMS.
Hyperglycemia is common in critically ill patients, appearing in 90% of them during serious illness, were diabetic or not before admission. It occurs as an adaptive response to aggression to ensure delivery of glucose to the tissues in serious situation.1–3 The most recent reports have shown that uncontrolled hyperglycemia has an adverse effect on mortality of critically ill patients.4–9 In this setting there is a resistance to insulin action of multifactorial origin, which makes difficult the control of blood glucose. For this reason, high doses of insulin can be needed, with the resultant risk of hypoglycemia.
In 2001, Van den Berghe et al. described as the strict control of blood glucose decreased morbidity and mortality in critically ill surgical and, in subsequent studies, also in medical patients.10–12 The benefits obtained with this control need to maintain blood glucose in the range of 4.44–6.1mmol/l (80–110mg/dl), administering insulin intravenously in most cases.
More recent studies shown that strict control of blood glucose may not be beneficial or may even get worse in the prognosis of patients, due to an increase in late mortality. The main difference in complications that appeared in the strict control group compared to the control group of less strict glycemic is the occurrence of severe hypoglycemia, which may be associated with severe morbidity and mortality.13–15
Monitoring of capillary blood glucose has been customary in the ICU for adjustment of insulin requirements of patients, until recently. It is a simple procedure with few complications for the patient and is economical, with a good correlation with blood glucose in most patients. Studies of glycemic control in critically ill patients have been performed by measuring CG intermittently, with the risk of the existence of periods of hypoglycemia and hyperglycemia undetected between measurements. Besides the difficulty of detecting large glucose excursions, intermittent control of CG requires multiple punctures and an increase in the nurse staff workload.
Currently, continuous glucose monitoring is performed in diabetic outpatients by sensors positioned in the subcutaneous tissue, but these devices have not been incorporated into the routine monitoring in the ICU. The development of these devices of subcutaneous continuous glucose monitoring system emerged as a need for close monitoring of blood glucose concentrations in patients with metabolic instability or insulin pumps carriers, thereby reducing the risk of complications. These devices were first developed in the 1980s and its operation is based on subcutaneous implantation of a sensor carrying an enzyme electrode measuring interstitial glucose concentration. The device security is very high, as it requires no more than a small subcutaneous implant, whose placement is almost painless, the measurement effectiveness in an outpatient being 100%, although there are certain problems that can reduce their effectiveness, as are the limited lifetime of the sensor (96h), the need for calibration (at least 1 time every 12h), need for change of anatomical site of implantation and the risk of infection from the puncture site. The validity of its values assumes a constant relationship between plasma and interstitial fluid glucose across the range of plasma glucose values.16 A limited number of reports on its use in critically ill patients have been published, yielding different results, although most show a good correlation between the values obtained with the CGMS and intermittent glycemia conventionally obtained.17–28
Our CGMS study was performed in patients with distributive shock, patients with multiple factors that hinder glycemic control. Our aim was to assess the reliability of measurements obtained by a subcutaneous enzyme sensor, in such patients whose peripheral perfusion and metabolism may be greatly affected by hypoperfusion, mediators of inflammation and drugs administered, altering the intracellular uptake of glucose.
In our study we proceeded to assess the correlation between tissue and capillary blood glucose continuous glucose obtained intermittently in patients with distributive shock who required intravenous insulin infusion to control capillary blood glucose in the presence of distributive shock.
Materials and methodsPatients 18 years or older admitted to the intensive care unit (ICU) between September 2010 and September 2011 were considered for the study. To be included, they had to be diagnosed of a cause of distributive shock and to require intravenous insulin infusion for glycemic control. The diagnosis of distributive shock was made excluding other causes of shock (hypovolemia, haemorrhagia, cardiogenic shock, neurogenic shock), in the presence of systolic blood pressure less than 90mmHg or at least 30mmHg lower than their usual systolic pressure, after an initial crystalloids load of a least 30ml/kg.
In addition to treating the disease that caused the ICU admission, in the selected patients we placed them a sensor for continuous measurement of subcutaneous tissue glucose level model Medtronic MiniMed Soft-Sensor™ glucose sensor (Medtronic MiniMed, California, USA), holding up to 120h, to perform a CGMS. The sensor makes up to 288 glucose measurements for 24h (1 measurement every 5min). The CGMS sensors were placed in the lateral abdominal wall, in an area with absence of skin lesions and the greatest possible distance from surgical incisions or soft tissue infections if any. The subcutaneous sensor carries a membrane which is coupled to the enzyme glucose oxidase and is placed on an amperometric sensor which is able to respond linearly to glucose in the range of 2.22–38.9mmol/l (40–700mg/dl). The data are then sent through a radio receiver and downloaded to a computer for analysis. The data obtained by the CGMS were processed using the CareLink Software-pro™ (Medtronic MiniMed, California, USA) for Windows™ (Microsoft Corp. One Microsoft Way, Washington, USA). Data were then incorporated into a database for analysis. The calibration of the CGMS was performed every 8h, using the result of CG measurement. Pairs of values corresponding to calibration of the CGMS were not taken into account for statistical analysis.
The CG monitor used during the study was the Optium Xceed™ system (Abbott Diabetes Care Ltd., Witney, UK). According to the features provided by the manufacturer, it has an accuracy of 3–3.6% and a reliability of 98% compared to capillary samples, 99% versus venous samples and of 97% versus arterial samples. The monitor complies with ISO 15197 rules. Its range of monitor readings is between 1.1 and 27.8mmol/l (20mg/dl and 500mg/dl) for a hematocrit between 20% and 60%. The reference samples are capillaries obtained by finger prick. Glucose in the sample reacts with nicotinamide adenine dinucleotide (NAD) requiring glucose dehydrogenase (NAD-GDH) on the test strip. The minimum volume required is 2.5μl of blood and the time to obtain the result is 20s.
We compared the results obtained with both methods of glucose measurement obtained in the first 72h after the placement of the CGMS sensor. At the time of the study, the manufacturer recommended not to extend their use beyond this period; following the completion of the study, up to 120h use has been accepted.
Regardless of device implantation, patients followed the approved protocol for glycemic control in the ICU. The measurements of CG, insulin infusion and artificial nutrition were administered according to this protocol compliance. The glycemic control protocol aims to maintain the range of blood glucose of patients between 5.56 and 7.78mmol/l (100–140mg/dl). The patient's blood glucose level indicates the frequency of monitoring, performing every 30min in patients with hypoglycemia, to be held every 4h in patients with maintained stability of target glycemia. Intravenous insulin infusion was started to all patients not taking oral diet alone (in this setting, insulin was administered by subcutaneous injection), who presented two consecutive blood glucose measurements greater than 7.78mmol/l (140mg/dl), separated by 4–6h. Patients leave the insulin infusion protocol when they do not need insulin infusion to maintain blood glucose <140mg/dl or when starting oral diet. Medical and nursing staff remained unaware of CGMS data records; therefore, these data were not used to make changes in insulin treatment.
In addition to CGMS and CG measurements, demographic data (age, gender, weight, height, body mass index, previous diagnosis of diabetes), cause of distributive shock, APACHE II score of the first 24h, SAPS III score, daily insulin needs, nutritional daily caloric intake, doses of vasopressors drugs, and the use of corticosteroids were recorded.
Statistical analysisResults are presented using absolute frequencies and percentages when categorical variables are shown and as mean, median, maximum, minimum and standard deviation (SD) in the case of quantitative variables. A descriptive analysis was performed on the data set so as to calculate the absolute difference and the relative difference to each reference pair (difference between CGMS measurements and CG measurements) and among means of glucose values obtained by both methods. To quantify the correlation and variability we used the Pearson correlation coefficient and the intraclass correlation coefficient (ICC) for the whole group. The results were interpreted according to the criteria of Landis and Koch.29 Agreement between values was assessed using the Bland–Altman method, modified by Krouwer.30,31 To assess the correlation between the two analytical methods, the nonparametric regression of Passing–Bablok was employed.32
Statistical analysis was performed using the SPSS 15 software for Windows™ (SPSS Inc., Chicago, USA) and MedCalc for Windows™ Version 12.4.0 (MedCalc Software, Ostend, Belgium).
The present study was approved by the Committee on Clinical Trials and Research of our institution. Written informed consent for study inclusion was obtained from patients or from their legal representatives. The study has been performed in accordance with the ethical standards from the 1964 Declaration of Helsinki and its later amendments, as well as with local laws.
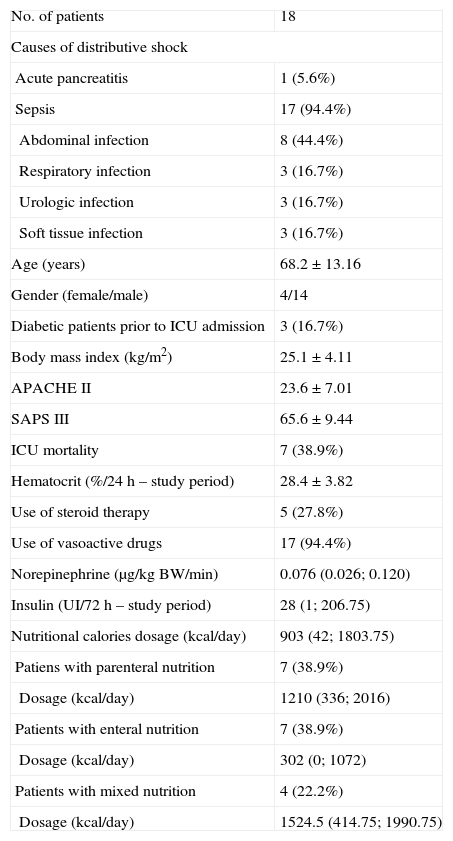
ResultsTwenty-three patients admitted consecutively to the ICU, fulfilling the inclusion criteria, were included in the study. Five of them were subsequently excluded due to the inability to obtain measurements of CGMS. Demographic characteristics and other variables from the 18 patients who eventually formed part of the study are shown in Table 1.
Baseline characteristics.
| No. of patients | 18 |
| Causes of distributive shock | |
| Acute pancreatitis | 1 (5.6%) |
| Sepsis | 17 (94.4%) |
| Abdominal infection | 8 (44.4%) |
| Respiratory infection | 3 (16.7%) |
| Urologic infection | 3 (16.7%) |
| Soft tissue infection | 3 (16.7%) |
| Age (years) | 68.2±13.16 |
| Gender (female/male) | 4/14 |
| Diabetic patients prior to ICU admission | 3 (16.7%) |
| Body mass index (kg/m2) | 25.1±4.11 |
| APACHE II | 23.6±7.01 |
| SAPS III | 65.6±9.44 |
| ICU mortality | 7 (38.9%) |
| Hematocrit (%/24h – study period) | 28.4±3.82 |
| Use of steroid therapy | 5 (27.8%) |
| Use of vasoactive drugs | 17 (94.4%) |
| Norepinephrine (μg/kg BW/min) | 0.076 (0.026; 0.120) |
| Insulin (UI/72h – study period) | 28 (1; 206.75) |
| Nutritional calories dosage (kcal/day) | 903 (42; 1803.75) |
| Patiens with parenteral nutrition | 7 (38.9%) |
| Dosage (kcal/day) | 1210 (336; 2016) |
| Patients with enteral nutrition | 7 (38.9%) |
| Dosage (kcal/day) | 302 (0; 1072) |
| Patients with mixed nutrition | 4 (22.2%) |
| Dosage (kcal/day) | 1524.5 (414.75; 1990.75) |
Values are expressed as absolute rates and percentages and as mean value±standard deviation or median and interquartile range.
The cause of distributive shock was sepsis in 17 patients (94%) and pancreatitis in 1 patient. Seven patients (38.9%) received parenteral nutrition alone, 7 patients (38.9%) enteral and 4 patients (22.2%) received both simultaneously. Seventeen patients (94%) required the use of vasopressor drugs, mostly noradrenaline (17 patients, 94.4%), with administration of dopamine and dobutamine in only two patients. One patient required three types of vasopressor drugs. The hematocrit remained in all patients between 20% and 60% along the study period.
Finally, 11,673 CGMS and 348 CG values were obtained, with 295 paired. Mean CGMS value was 7.45mmol/l (134.07mg/dl) (SD 39.62) and mean CG value was 7.82mmol/l (140.70mg/dl) (SD 40.12). Among values of CGMS, 1934 (16.57%) were consistent with measurements <5.56mmol/l, and 4619 (39.57%) with values >7.78mmol/l, corresponding with the target blood glucose range (5.56–7.78mmol/l) 5120 (43.86%) values. Forty-seven CG values (13.51%) were <5.56mmol/l, 162 (46.55%) were >7.78mmol/l and 139 (39.94%) were within the target range of protocol.
Glucose values <3.33mmol/l (<60mg/dl) were obtained in 42 measurements (3.6%) recorded by CGMS, without any severe hypoglycemia (<2.22mmol/l; <40mg/dl); CG values showed a single episode of glucose <3.33mmol/l (0.29%), 2.28mmol/l being the lowest recorded value.
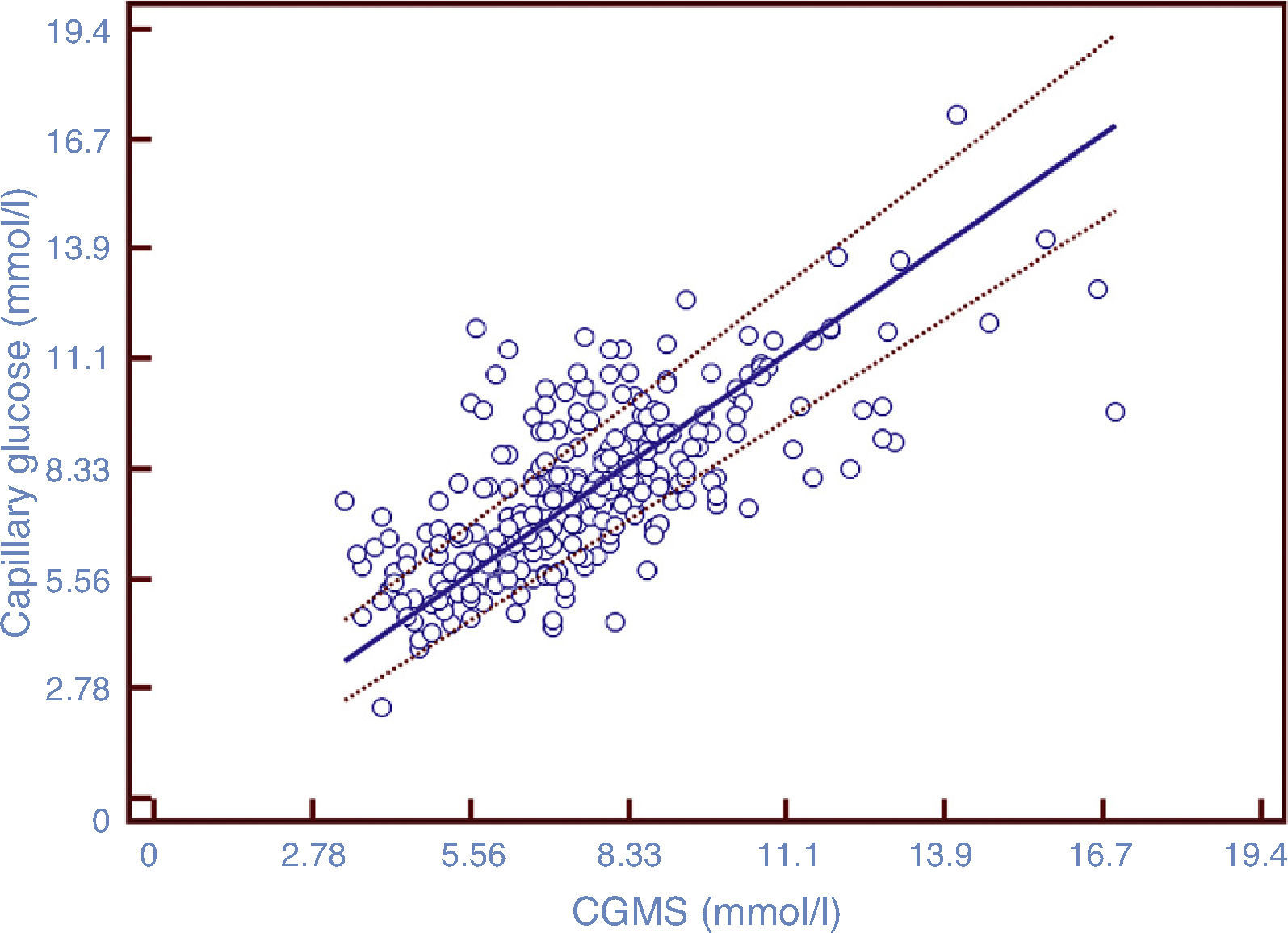
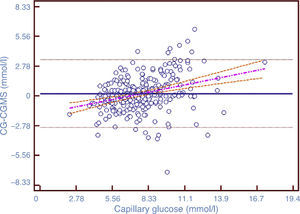
After comparing the values of CGMS and CG, the ICC was 0.706, showing a substantial degree of agreement on the scale proposed by Landis and Koch.29 Of the total variability, 29.4% was due to the method used for glucose measurement. The Pearson correlation coefficient was 0.71 (p<0.0001, 95% CI 0.65–0.76). Fig. 1 shows the positive linear correlation between CGMS and CG values using the regression method of Passing–Bablok.
Scatterplot of paired data. The solid line represents the regression line; the dotted lines represent the confidence interval of 95%. Correlation coefficient=0.71 (p<0.0001), 95% CI 0.65–0.76. The figure shows the existence of a positive linear correlation between the two methods of measurement.
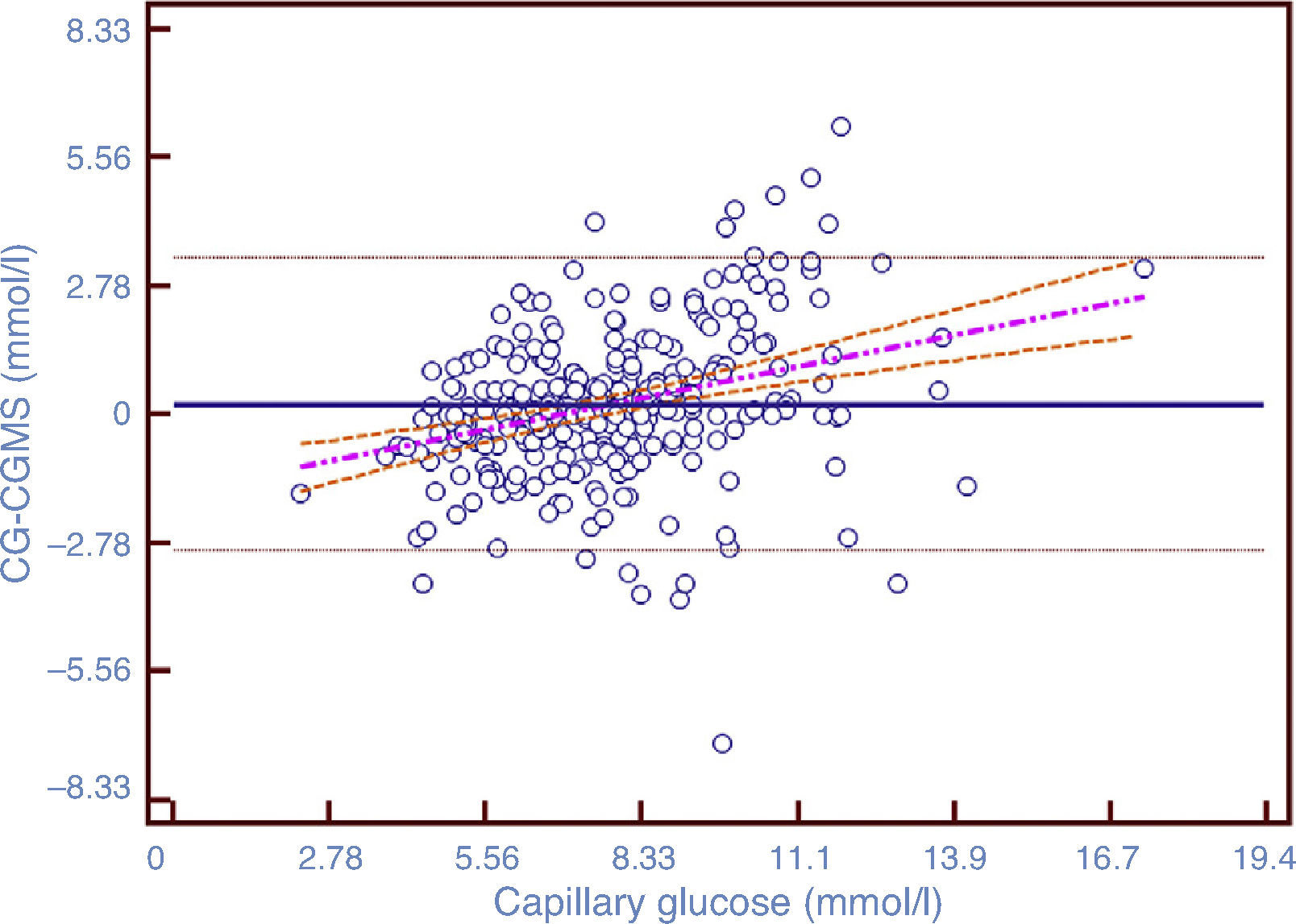
Mean difference between the 295 paired measurements is shown in a Bland–Altman analysis (Fig. 2). There is a tendency of the CGMT with respect to CG to overestimate blood glucose in the high blood glucose ranges and underestimate in the low range, with normalization in the range of normoglycemia. In 95% of the measurements the difference from the GC is ±1.61mmol/l (29mg/dl), which is lower difference in the 5.55–8.33mmol/l (100–150mg/dl).
Bland–Altman plot modified by Krouwer. In this case the differences between the two methods of measurements are plotted against the CG, considered as the reference method in this study. The black line represents the bias between both methods of measurement, and the black dotted lines represent ±1.96 SD. The mean difference (bias) is 3.98±29.04mg/dL. The dashed pink line represents the correlation between the two methods. The orange dotted line shows 95% CI.
In our study, no complications related to the insertion of the subcutaneous device is presented, showing its safety when it is inserted following the deployment instructions and with appropriate aseptic precautions.
DiscussionTight control of blood glucose levels in the critically ill patients, its impact on morbidity and mortality and the blood glucose range established as beneficial and safe have been the subject of numerous studies in the last decade.
Leuven studies 1 and 2 compared a strict protocol of glycemic control in critically ill patients, defined as the goal of maintaining blood glucose level between 4.44 and 6.11mmol/l (80–110mg/dl), with conventional control, which tried to maintain blood glucose between 10 and 11.11mmol/l (180–200mg/dl).10,11 The results from both studies showed that in the groups subjected to strict control of blood glucose was decreased mortality in surgical patients (with low probability of death), without reducing it in short-stay medical patients. A subsequent intention to treat analysis, including patients from both studies, showed a reduction in mortality and morbidity when tight control was performed for at least three days, as well as that tight blood glucose control was not harmful if its duration took less than three days, that the result was independent of initial glucose load and that there was no benefit of tight blood glucose control in diabetic patients.33
The glycemic threshold that could help reduce mortality may be over the value assessed by Van den Berghe et al. in the above studies.10,11 The study of Krinsley showed a decrease in hospital mortality, length of ICU stay, renal failure and transfusion requirements in medical–surgical patient with an insulin infusion protocol in order to maintain blood glucose <7.78mmol/l.12 It has not been clearly established to date which patients might benefit from tight control of blood glucose, being even possible that this practice to be harmful to patients with brain injury.34,35
The most important concern about a strict control of blood glucose is hypoglycemic episodes, especially most severe (blood glucose level below 2.22mmol/l) which can be related to increased mortality.13 In fact, the results from the recently published NICE-SUGAR study showed that 82% of moderate hypoglycemia and 93% of those severe appeared in the strict blood glucose control group. There was an increased mortality rate in patients with moderate (OR 1.41, 95% CI 1.21–1.62, p<0.001) and severe hypoglycemia (OR 2.10, 95% CI 1.59–2.77, p<0.001). Mortality increased in patients with repeated hypoglycemia (more than one episode of hypoglycemia per day), in patients with distributive shock and in those with severe hypoglycemia in the absence of insulin.14,36 Our results show that CGMS can detect blood glucose <3.33mmol/l at a rate 12.4 times greater than CG (glucose values <3.33mmol/l recorded by CGMS and by CG, 3.6% and 0.29% respectively), although these data could be altered by the greatest number of values obtained by the CGMS, being part of the recorded data pertaining at the same episode.
Therefore, continuous monitoring of glucose is an attractive method to prevent hypoglycemic episodes, while maintaining a desirable blood glucose range in critically ill patients, who are subjected to multiple causes of significant variability in glycemia. The increase in this variability has also been associated with mortality and may be modified by a continuous monitoring system.37
In addition, one of the main advantages of the CGMS is the ability to recognize trends in the patient's blood glucose under insulin treatment, allowing an early reaction, even before the disturbance occurs (hypoglycemia or hyperglycemia), and decreasing the onset of serious complications associated with consequences of morbidity and mortality.
CGMS, used initially in diabetic outpatients, has been proven to be safe and reliable in critically ill patients with different admission diagnoses, when compared with plasma glucose measurements.18,22,23,25–27 We have compared CGMS values obtained by a device to the patient's bedside, with CG values. Systems measuring capillary whole blood glucose have an acceptable reliability and accuracy, with a good correlation, when compared to plasma glucose, allowing fast results and avoiding high blood volume samples.19 Therefore, we believe that it is appropriate to make the comparison of values obtained by CGMS with commonly employed glucose determination systems. Our study showed a positive linear correlation between the two measurement methods (Fig. 1), with a proportional error in the extreme values, without clinical relevance since the mean of the differences of values reached 0.22mmol/l (3.98mg/dl) (Fig. 2).
For our study, we selected patients with distributive shock because these patients have a very difficult glycemic control as a result of the inflammatory response, insulin resistance and erratic caloric intake.
Our study has two fundamental limitations which are the small sample size of patients included in the study, although partly offset by the large number of measurements obtained from CGMS, higher than in previous studies, and the other the loss of patients due to incapacity sensing device CGMS. Five patients had to be excluded due to inability to obtain measurements, despite changing the sensor insertion site. The study was not designed to give an explanation for this finding, neither is it an analysis of subgroups. The absence of sensing could be motivated by the presence of one or more alterations in subcutaneous tissue, such as impaired microcirculation, temperature variability or subcutaneous tissue edema.22
To our knowledge, ours is the first report comparing the accuracy and reliability CGMS values with CG values in patients with distributive shock, and the first to report the inability to obtain CGMS measurements in some patients. A paper published recently by Holzinger et al., comparing the measurements obtained with the same CGMS that we used in our study with arterial blood glucose, in patients with and without shock (some of them requiring norepinephrine), did not detect any influence of these variables on the accuracy and reliability of the measurements obtained with this subcutaneous sensor.24 In another study, the same authors reported that CGMS reduced the absolute risk of severe hypoglycemia by 9.9% in critically ill patients with very different diagnosis, including septic shock.38 In addition, CGMS values have shown a better accuracy in patients with septic shock than in patients with other serious illnesses, when compared with arterial blood glucose.28
ConclusionsWith the data obtained we observe that when the CGMS is able to collect data, there is a correlation between the values obtained by this and capillary blood glucose in patients with distributive shock.
The use of continuous glucose sensors tissue in ICU may benefit patients with distributive shock, because a more precise monitoring is obtained, enabling early diagnosis of the presence of glucose excursions (hypoglycemic and hyperglycemic) facilitating compliance with protocols of insulin infusion, alerting us of changes in the metabolic state of the patient, obtaining possibly decreased morbidity associated with the strict glycemic control.
There remains the problem of the lack of sensing in patients in shock, which could be overcome with the development of intravascular glucose sensors continuously.
All this should be confirmed in further studies with larger numbers of patients, preferably using as a control blood glucose levels.
Until then, the use of CGMS in patients with distributive shock can be assessed, because there is a high percentage of patients who may benefit from their use, without complications arising from their use. Also, the costs do not rise significantly because they are economic devices that work for a long time (up to 5 days), reducing the workload of nurses, although this has not been evaluated.
Competing interestsThis study has received the financial support of a Cohesion Fund grant from the Health Ministry of Spain, in the year 2009.
The authors declare neither to have received any grant or financial support from the manufacturer of the device assessed in the study, nor to have any conflict of interest regarding this study.
Our gratitude to nursing staff of the Intensive Care Department, for their help in obtaining samples and their cooperation during the study.