Deep sedation during stay in the Intensive Care Unit (ICU) may have deleterious effects upon the clinical and cognitive outcomes of critically ill patients undergoing mechanical ventilation. Over the last decade a vast body of literature has been generated regarding different sedation strategies, with the aim of reducing the levels of sedation in critically ill patients. There has also been a growing interest in acute brain dysfunction, or delirium, in the ICU. However, the effect of sedation during ICU stay upon long-term cognitive deficits in ICU survivors remains unclear. Strategies for reducing sedation levels in the ICU do not seem to be associated with worse cognitive and psychological status among ICU survivors. Sedation strategy and management efforts therefore should seek to secure the best possible state in the mechanically ventilated patient and lower the prevalence of delirium, in order to prevent long-term cognitive alterations.
La sedación profunda durante la estancia en una Unidad de Cuidados Intensivos (UCI) puede afectar negativamente al estado clínico y cognitivo de los pacientes críticos sometidos a ventilación mecánica. En la última dèc)cada ha aparecido gran cantidad de literatura sobre diferentes estrategias dirigidas a reducir los niveles de sedación en el paciente crítico. Además, ha aumentado el interèc)s sobre la disfunción cerebral aguda o delirium. Sin embargo, el efecto de la sedación sobre los dèc)ficits cognitivos a largo plazo continúa siendo poco conocido. Las estrategias centradas en reducir los niveles de sedación en UCI no parecen estar asociadas con un peor estado cognitivo y psicológico de los supervivientes. Por lo tanto, las estrategias de manejo de la sedación en UCI deberían focalizarse en mejorar el estado del paciente ventilado, así como en disminuir el delirium, con el fin de prevenir las alteraciones cognitivas a largo plazo.
Sedative and analgesic agents are widely used by physicians to treat pain, stress and discomfort in critically ill patients admitted to Intensive Care Units (ICUs). During the late 1990s, ICUs worldwide developed a culture of very deep and prolonged sedation and paralysis, especially in patients requiring vital support techniques such as mechanical ventilation (MV).
However, sedation may also have deleterious effects. Several negative short- and long-term outcomes have been associated with increased levels of sedation in medical and surgical ICU patients undergoing MV. The administration of sedative agents may produce respiratory depression, hemodynamic instability or metabolic acidosis, and can prolong mechanical ventilation and ICU stay and increase the likelihood of the development of delirium.1,2
Over the last decade there has been an increasing interest in the study of acute brain dysfunction, or delirium, in ICU patients.3•5 This growth can be attributed to the impact of delirium on clinical outcomes in critically ill patients on MV, including increased mortality,6,7 prolongation of MV and hospital stay,8 higher costs of care,9 and long-term cognitive impairment.10 Various sedative agents have been identified as likely predictors of the development of delirium in mechanically ventilated ICU patients, suggesting a link between sedation and critical illness-associated brain dysfunction.11•13 However, outside the context of delirium, the contribution of sedation to long-term brain dysfunction in critically ill patients has not been discussed or comprehensively evaluated.14 Therefore, the aim of this review is to explore the role of sedative dosing strategy in the development of neurocognitive dysfunction after ICU stay.
Clinical outcomes and sedation strategyThe current trend in patients undergoing mechanical ventilation in the ICU is to moderate the depth of sedation. This procedure has been promoted by clinical trials that have indicated the need for lower levels of sedation in critical care and have implemented a variety of strategies including daily sedation interruption, goal-directed sedation, or even no sedation at all.15
The daily sedation interruption strategyDaily sedation interruption is defined as a short-term suspension, holding, discontinuation, or cessation of intravenous sedation or (in some cases) analgesic medication.16 The first clinical trial using this sedation strategy16 concluded that daily interruption of the infusion of sedative drugs was a safe and practical strategy to treat ICU patients undergoing MV which also improved clinical outcomes, decreasing the duration of MV and shortening ICU stay. To test whether lower sedation doses in ICU patients might affect the long-term psychological status of ICU survivors, a small sample of the study cohort was monitored for psychological symptoms17; at six months, no significant differences between groups were observed for anxiety, depression and functionality. However, patients in the intervention group had a lower Impact of Event Score (p=0.02), suggesting that the daily sedation interruption strategy was beneficial rather than harmful and reduced symptoms of post-traumatic stress disorder.
Since that study, several trials have explored the effect of daily sedation interruption on clinical outcomes in ICU patients.18 Daily spontaneous awakening trials seem to reduce time in coma, ICU and hospital length of stay, sedation and to increase time off MV, and the 1-year survival rates.19,20 Although other authors did not find significant improvements in different clinical and psychological outcomes,21•23 daily sedation interruption has been recommended by the Society of Critical Care Medicine guidelines in order to achieve light levels of sedation in mechanically ventilated ICU patients.11
The goal-directed sedation strategyThe impact of deep sedation during the first 48hurs of ICU admission on the short- and long-term clinical outcomes was investigated by Shehabi et al. In two different studies, the authors showed that, after adjusting for illness severity and other confounders, early deep sedation was an independent predictor of long-term mortality and time to extubation in mechanically ventilated ICU patients. Although early deep sedation and the cumulative dose of sedative agents were not associated with time to delirium after 48h, lightly sedated patients had a lower presence of delirium at 48h,24 as well as significantly more coma- and delirium-free days at 28 days.25
In the light of these findings, the authors proposed a sedation algorithm termed early goal-directed sedation. This process is implemented early after initiation of mechanical ventilation, is goal-directed to target light levels of sedation whenever possible, and uses dexmedetomidine as the primary sedative agent, thus minimizing the use of benzodiazepines.26 Surprisingly, the new process did not show significant benefits in terms of MV duration, ICU/hospital length of stay or mortality when compared with standard care; nor did the duration of ICU delirium improve significantly, although the early goal-directed sedation group had more delirium-free days, received significantly less benzodiazepine or propofol, and required significantly less physical restraint. Therefore, the early goal-directed sedation strategy needs further investigation to clarify its impact on clinical outcomes during ICU stay. Even so, the results obtained so far draw attention to the unnecessary use of benzodiazepines in the ICU and underline the positive effect of avoiding early deep sedation on the mental status and well-being of critically ill patients.
The no sedation strategyStrom et al.27 aimed establishing the impact of the application of a no sedation protocol versus daily interruption of sedation on the duration of MV in critically ill patients. Patients under the no sedation protocol had more MV-free days and shorter ICU and hospital stays. However, agitated delirium was more frequent (20% vs. 7%) in the no sedation strategy group, in which the use of haloperidol was significantly increased. The results of this study suggest the striking idea that the no sedation strategy may be beneficial for certain clinical outcomes such as duration of MV or ICU/hospital stay, but not for the occurrence of ICU delirium. Nevertheless, it should be borne in mind that applying a no sedation strategy increases awareness in ICU patients and that delirium cannot be masked under sedation. Therefore, the higher rates of delirium in the no sedation strategy group may reflect better detection and diagnosis of the acute ICU brain dysfunction rather than a relationship between the non-use of sedative agents and ICU delirium.
Choosing the best sedative strategy for the ICU patients may be context-specific and may depend on the clinical population. However, the consistent message from the literature is that, if possible, minimizing sedation in critically ill patients undergoing MV may be beneficial.28 It seems clear that reducing or even avoiding sedation during the ICU stay does not have a detrimental effect on critically ill patients; in fact, keeping ICU patients lightly sedated allows clinicians to reduce the use of benzodiazepines and sedative agents and thus avoid the associated adverse events. The improvement inpatients tm) awareness and well-being permits clinical staff to examine their mental state more closely and to achieve a more accurate diagnosis of delirium. We should also bear in mind the potential benefit of a reduction in sedation for the different clinical outcomes during the ICU stay, as well as for survival rates and the psychological status of the ICU survivors.
Impact of sedation in the acute brain dysfunction (delirium) in ICUDelirium is understood as an acute form of brain dysfunction that affects 14•24% of hospital admissions and 15•53% of postoperative patients.29 In the critical care context, the prevalence of delirium rises to between 60% and 80% in ICU patients undergoing MV,6,12,30,31 and delirium duration has emerged as an independent predictor of mortality, ventilation time, ICU length of stay6,32 and short- and long-term cognitive impairment10 in critically ill patients and ICU survivors. Furthermore, its presence has been associated with a 39% increase in ICU costs.9 Thus, delirium can be considered as the first manifestation of cognitive impairment in critically ill.
The list of risk factors for delirium in ICU patients is extensive and heterogeneous.33•36 In general, the risk factors associated with the presence and duration of delirium in ICU are classified into two types of predisposing factors (patients tm) characteristics and chronic pathology) and two types of precipitating factors (environment and acute illness status). The precipitating factors are generally considered to be the more modifiable. All the studies and reviews suggest that the management of sedation and analgesia during the critical illness influences the prevalence and duration of delirium in ICU patients. Even in critically ill patients undergoing MV in which clinically-induced coma is required for life-support, avoiding the over-use of the medications should be considered. Although preliminary, current data suggests that the time in burst suppression detected by the bispectral index (BIS) during depth sedation could be an independent predictor of the occurrence and duration of the ICU delirium.37 Thus, since sedative and analgesic treatment is included among the precipitating factors, the management of pharmacological interventions during the ICU stay should be regarded as a good target for the prevention of delirium in critically ill patients.38,39
Sedative agents, sedation strategies and deliriumThe influence of benzodiazepine administration on the development of delirium in ICU patients is well documented in the literature.1,14 Midazolam and lorazepam, the benzodiazepines most commonly used in critical care, have been associated with longer ICU and hospital stays and also increased MV time in comparison with non-benzodiazepine treatment.1,40•43 Short-acting agents such as propofol, dexmedetomidine or remifentanil can be rapidly adjusted and their use can help to minimize the depth and duration of sedation with a potential reduction of time to extubation and days of delirium in the ICU.28,42,43 As a result, non-benzodiazepine treatment, such as dexmedetomidine, may be expected to reduce and even prevent the duration and incidence of delirium in critically ill patients.44 Due to its sedative and analgesic effects, with a little interaction with other drugs, fewer side effects, and an easy titration, the use of dexmedetomidine could be a better alternative to deliriogenic sedatives and haloperidol or other atypical antipsychotics. Although to date more evidence is needed, preliminary data suggest that the use of dexmedetomedine might be effective preventing and treating agitated delirium during the ICU25,42,43,45 in both mechanically intubated46 and nonintubated critically ill patients.47
Unlike the effect of individual sedative agents, the impact of each sedative strategy on ICU delirium has not been explored in depth. In one study, the incidence of delirium in ICU patients undergoing protocolized sedation did not decrease when daily sedation interruption was added.22 Similar results were found in patients managed with a daily spontaneous awakening trial (SAT) followed by a spontaneous breathing trial (SBT) or with sedation per usual care plus a daily SBT.19 Decreasing sedation in the first two days of the ICU stay improved the incidence of delirium in mechanically ventilated patients, although early deep sedation or the cumulative dose of sedative agents did not predict the time to delirium after 48h.24,25 Finally, no sedation strategies may lead to higher rates of agitated delirium,27 although these results may also reflect the under diagnosis of delirium in sedated ICU patients.
Sedative/analgesic drugs during ICU stay and the long-term cognitive outcomesThe direct relationship between the use of sedative agents and cognitive outcome in ICU survivors has not been widely studied. In fact, only two studies have considered the specific hypothesis that higher doses of sedative and/or analgesic agents may be associated with cognitive impairment after hospital discharge (Table 1).
Studies whose main objectives include analysis of sedation and cognition.
| Authors | Year of publication | Objective/Hypothesis | Sample and inclusion/exclusion criteria | Sedation strategy | Sedative/analgesic agents | Assessed Domains | Results |
|---|---|---|---|---|---|---|---|
| Jackson et al. | 2010 | To determine the long-term effects (neurocog, psychological and functional) of a wake up and breathe protocol that interrupts and reduces sedative exposure in the ICU | 180 Medical ICU>12h of MV CP arrest & neurocriticals excluded | Two randomized groups: (1) SATs+SBTs (2) Usual care group: patient-targeted sedation+SBT protocol | Preenrollment sedative exposure • Lorazepam equivalents, mg • Fentanyl equivalents, mg • Propofol Sedative exposure during trial • Lorazepam equivalents, mg • Fentanyl equivalents, ug • Propofol | • Neurocognition • PTSD • Anxiety • Depression • Functionality (comprehensive battery) | SATS+SBTs group vs. Control group Exposure to benzodiazepines (lorazepan equivalents, 21mg • 5•83 vs. 42mg • 10•296; p=0.04) Exposure to propofol (5.070α/4g • 2.290•8.825 vs. 2.600•1.310 • 7.395; p=0.04) At 3 months: SAT+SBT group vs. Control group Cognitive impairment (70% vs. 91%; p=0.03) Depression (64% vs. 58%; p=0.59) Post-traumatic stress (14% vs. 10%; p=0.59) Functional status reported (72% vs. 74%; p=0.84) At 12 months: SAT+SBT group vs. Control group Cognitive impairment (72% vs. 70%; p=0.89) Depression (59% vs. 62%; p=0.82) Post-traumatic stress (24% vs. 24%; p=0.97) Functional status reported (64% vs. 87%; p=0.05) |
| Pandharipande, et al. | 2013 | A longer duration of delirium in the hospital and higher doses of sedative and analgesic agents are independently associated with more severe cognitive impairment up to 1 year after hospital discharge | 821 Medical or surgical ICU with respiratory failure, cardiogenic shock or septic shock Short- IQCODE ≥3.6 and CDR >2, excluded | No specific sedation strategy reported. | • Benzodiazepine • Propofol • Dexmedetomidine • Opiate | • Delirium (CAM-ICU) • Neurocognition (RBANS) • Executive functions (TMT B) | Delirium during hospital stay in 74% of the patients (median 4 days) At 3 months: Patients with cognitive status below 1.5 standard deviations: 40% Duration of delirium independent risk factor for: • Global cognitive impairment (p=0.001) • Executive dysfunction (p=0.004) Higher benzodiazepine dose independent risk factor for: • Executive dysfunction (p=0.04) At 12months: Patients with cognitive status below 1.5 standard deviations: 34% Duration of delirium independent risk factor for: • Global cognitive impairment (p=0.04) • Executive dysfunction (p=0.007) |
MV=mechanical ventilation; SAT=spontaneous awakening trials; SBT=spontaneous breathing trials; PTSD=post traumatic stress disorder. Short-IQCODE=Short-Informant Questionnaire on Cognitive Decline in the Elderly. CDR=Clinical Dementia Rating; CAM-ICU=Confusion Assessment Method for the ICU; RBANS=Repeatable Battery for the Assessment of Neuropsychological Status; TMT-B=Trail Making Test, part B.
Jackson et al.48 followed medical ICU patients from the Awakening and Breathing Controlled Trial19 at 3 and 12 months after hospital discharge. Only two significant differences were found in the clinical outcomes during the ICU stay between the daily spontaneous awakening trials (SAT)/spontaneous breathing trials (SBT) protocol group and the usual care (patient-targeted sedation and an SBT) protocol group: the SAT/SBT group had higher propofol exposure before enrollment than the usual care group and reduced exposure to benzodiazepines during the trial. At follow-up, both groups showed similar cognitive, psychological and functional outcomes. Cognitive impairment was present in 79% of all patients evaluated at 3 months and in 71% at 12months, but it was significantly less frequent in participants in the SAT/SBT group at the 3-month follow-up. Moreover, fewer patients in this group reported worse overall functional status at 12-month follow-up than before their critical illness. In view of these results, the authors concluded that interrupting or reducing sedation in the ICU improved short-term cognitive outcomes and long-term perception of functional status and did not increase the risk of adverse cognitive, psychological, or other outcomes.
In a multicenter prospective cohort study exploring medical or surgical ICU patients, Pandharipande et al.49 hypothesized that longer duration of delirium during ICU stay and higher doses of sedative and analgesic agents would be independently associated with more severe cognitive impairment up to 1 year after hospital discharge. In that study, delirium was assessed through the administration of the Confusion Assessment Method for the ICU (CAM-ICU). Daily doses of benzodiazepines, opioids, propofol and dexmedetomidine were recorded. As expected, delirium was an independent factor for a worse cognitive global score and reduced executive function after both 3 and 12 months of follow-up. However, higher benzodiazepine dose emerged as an independent risk factor only for worse executive function performance at 3 months of follow-up. One of the most striking conclusions of the study was that, one year after critical illness, one out of four patients had cognitive impairment similar in severity to that observed in mild Alzheimer's disease, and one out of three had a level of impairment typically associated with moderate traumatic brain injury. The authors concluded that this cognitive impairment is found ‘de novo tm) in the majority of patients and that there is an association between duration of delirium, worse long-term global cognition and decline of executive function. However, the lack of a significant relationship between sedative or analgesic medication and long-term cognitive impairment led the authors to interpret the results with caution, although they did not rule out an association between benzodiazepines and executive function at 3 months of follow-up; they suggested that any intervention (including an appropriate use of sedative agents) directed at reducing delirium may mitigate the brain dysfunction associated with critical illness. A larger randomized trial designed to compare the effect of a no sedation strategy with standard sedation management on the long-term cognitive function of ICU survivors is currently underway (ClinicalTrials identifier: NCT01967680).50
From other point of view, three studies have indirectly explored the relation of the sedation received during the ICU stay and the long-term cognitive sequelae of the critically ill survivors (Table 2).
Studies whose main objectives do not include analysis of sedation and cognition.
| Authors | Year of publication | Objective/Hypothesis | Sample and inclusion/exclusion criteria | Sedation strategy | Sedative/analgesic measure | Measures | Results |
|---|---|---|---|---|---|---|---|
| Jackson, et al. | 2003 | To examine neuropsychological function, depression, and quality of life 6 months after discharge in ICU patients who underwent MV | 275 (34 patients finally analyzed) Medical and coronary ICU patients Neurocritical patients, patients with mental retardation or psychiatric illness excluded | Not specified | • RASS (every 24h) | • Delirium (CAM-ICU) • Neurocognition • Anxiety • Depression • Quality of life (comprehensive battery) | At 6 months: One third of patients were impaired on neuropsychological testing at follow-up. No statistical differences were observed between impaired and non-impaired patients, although duration of delirium was slightly greater for the impaired group(4.5 vs. 4.2 days, p=0.24) and sedation scale scores during the ICU were lower (∧2.6 vs. ∧2.2, p=0.44) |
| Hopkins, et al. | 2005 | To characterize neurocognitive and emotional function and quality of life 1 year after hospital discharge in a prospectively identified cohort of ARDS survivors | 120 ARDS patients (74 patients finally analyzed) | Not specified | Days of sedatives Days of narcotics Days of paralytics | Primary outcomes at 1 and 2 years: Neurocognitive Total Score (comprehensive battery) Secondary outcomes at 1 and 2 years: Neurocognitive measures: • Verbal IQ • Performance IQ • Verbal Memory • Visual Memory • Attention/Concentration • Delayed recall Emotional state measures: • Depression • Anxiety QoL measure: • SF-36 | At discharge: The prevalence of neurocognitive sequelae in ARDS survivors was: 73% at hospital discharge Hypoxemia, but not days of sedation, was modestly correlated with attention, verbal memory and executive function deficits At 1 year follow-up: Prevalence of neurocognitive sequelae: 46% Prevalence of severe depression symptoms: 16% Prevalence of anxiety: 24% Improvement of QoL, due to physical amelioration Hypotension, but not days of sedation, was modestly correlated with memory impairment. At 2 year follow-up: Prevalence of neurocognitive sequelae: 47% Prevalence of severe depression symptoms: 23% Prevalence of anxiety:23% No changes in QoL |
| Girard et al. | 2010 | To demonstrate that duration of delirium in ICU is an independent predictor of long-term cognitive impairment after critical illness requiring MV | 77 mechanical ventilated ICU patients Neurocriticals, cardiopulmonary arrest, >2week of MV excluded | (1) SAT+SBT (2) Usual care group: patient-targeted sedation+SBT protocol | Total doses of benzodiazepines Total doses of opiates Total doses of propofol | Primary outcomes at 3 and 12 months: • Neurocognition (comprehended battery) Factors and confounders at 3 and 12 months: • Duration of delirium (days with positive CAM-ICU during 28 days) • Age • Years of education • Preexisting cognitive function (IQCODE Short-form) • Severity of illness • Severe sepsis • Total doses of sedatives | At 3 months: Cognitive impairment No impairment 21% Mild/moderate 17% Severe 62% Duration of delirium was the only independent predictor of cognitive impairment (p=0.02) after adjusting for age, education, preexisting cognitive function, severity of illness, severe sepsis, treatment group and total exposure to sedatives in the ICU At 12 months: Cognitive impairment No impairment 29% Mild/moderate 35% Severe 36% Duration of delirium was the only independent predictor of cognitive impairment (p=0.03) after adjusting for age, education, preexisting cognitive function, severity of illness, severe sepsis, treatment group and total exposure to sedatives in the ICU |
| Treggiari et al. | 2009 | To investigate whether light sedation favorably affects subsequent patient mental health compared with deep sedation | 129 Mixed ICU patients with MV Neurocriticals excluded Follow up 2 (4 weeks) R1-2=52/R3-4=50 | Two randomized groups: (1) Light sedation: Ramsay 1-2 (R1-2) (2) Deep sedation: Ramsay 3-4 (R3-4) | Ramsay scale (every 24h) RASS (every 24h) Cumulative doses (every24h) • Midazolam, mg • Propofol, mg • Etomidat, mg • Morphine equivalents, mg | Primary outcomes at 1 month: • PTSD • Anxiety • Depression | Light sedation group vs. Deep sedation group ICU discharge Cases of depression: 3 vs. 10; (p=0.02) Cases not evaluable due to cognitive impairment: 0 vs. 4; (p=0.04) Days of MV: 2.9 (5) VS. 5.5 (10.8); (p=0.02) ICU- free days: 4.9 (1•129) vs. 5.5 (2•99); (p=0.03) 1 month follow-up: PTSD score: 46 (29) vs. 56 (29); (p=0.07) |
MV=mechanical ventilation; RASS=Richmond Agitation and Sedation Scale; CAM-ICU=Confusion Assessment Method for the ICU; ARDS=acute respiratory distress syndrome; IQ=intelligence quotient; SAT=spontaneous awakening trials; SBT=spontaneous breathing trials; QoL=quality of life; IQCODE=Informant Questionnaire of Cognitive Decline in the Elderly; PTSD=post traumatic stress disorder.
From a different perspective, four other studies have indirectly explored the association between the sedation received during the ICU stay and long-term cognitive sequelae in critically ill survivors (Table 2). Aiming to examine cognitive and depressive status as well as quality of life 6 months after ICU discharge, Jackson et al.51 carried out a comprehensive assessment of 34 medical and coronary ICU patients. After adjusting for age, educational level and baseline dementia, the authors found that one-third of patients presented impairments on neuropsychological testing at follow-up. The cognitive impairments found in these patients were similar to those observed in mild clinical dementia. Determining the relationship between the clinical variables and the cognitive deficits was beyond the scope of the study; however, and although the differences did not reach statistical significance, more days with ICU delirium and a deeper level of sedation were observed in the cognitively impaired group.
Hopkins et al.52 followed a sample of acute respiratory distress syndrome (ARDS) patients for two years in order to determine the prevalence of neurocognitive impairment, emotional symptoms and quality of life. From the 74 patients included in the final analysis, 70% presented cognitive sequelae at hospital discharge, 45% at 1 year and 47% at 2 years. Hypoxemia and hypotension were modestly correlated with various cognitive domains such as attention, memory and executive functions at hospital discharge and at 1 year of follow-up, but not at 2 years. Nevertheless, no other clinical variables during the episode of critical illness, including days receiving sedative, narcotic or paralytic medications, were significantly associated with neurocognitive alterations in critically ill patients.
A subsample of the Awakening and Breathing Controlled Trial was studied by Girard et al.10 to determine whether duration of delirium was a predictor of long-term cognitive impairment among mechanically ventilated medical ICU patients. Nearly 80% of participants showed cognitive impairment at 3 months follow-up, and 61% at 12 months. After adjusting for age, education, preexisting cognitive function, severity of illness, severe sepsis, treatment group and total exposure to sedatives in the ICU, duration of delirium was the only independent predictor of short- and long-term cognitive impairment; in fact, between one and five days of delirium was associated with a 5-point decline on cognitive performance tasks at 3 months and a 7-point decline at 12 months.
Finally, in a randomized trial of the effect of light versus deep sedation on mental health after critical illness, mechanically ventilated ICU patients were assessed for post-traumatic stress disorder, anxiety and depression at hospital discharge and after four weeks of follow-up.53 The patients with lower sedative doses showed reductions of one day in the duration of MV and of 1.5 days in ICU stay, without an associated increase in adverse clinical events or adverse mental health effects. Although cognitive status was not considered as a main measure in the study, the authors observed that 6% of the patients in the deep sedation group could not be assessed at ICU discharge due to cognitive impairment, whereas all the patients in the light sedation group were evaluable. No significant differences were found between the groups in age, educational level or illness severity, or in hemodynamic, respiratory, and metabolic variables.
Examining the literature, it remains unclear whether sedation during the ICU stay may impact the long-term cognitive impairment of the ICU survivors. Nevertheless, the probable effect of deeper sedation states and higher doses of benzodiazepines during the ICU stay on the cognitive profile of post-critically ill patients in the short-term follow up should not be underestimated. After ICU and hospital discharge all cognitive domains may be affected, although the executive functions may be especially vulnerable to the aspects of sedation described above. More evident is the non-adverse effect of reducing ICU sedation on ICU survivors tm) cognition. No harmful effect on the cognitive and psychological status has been found in the literature when strategies aimed at keeping mechanical ventilated patients lightly sedated are applied. The same conclusion can be drawn when benzodiazepine use is reduced during the ICU stay.
How do we explain the relation between sedation and acute/short-term cognitive outcomes? The probable pathophysiological mechanismsThe pathophysiology of ICU delirium remains uncharacterized,28 although several hypotheses are being studied.54 The main difficulty lies in the wide range of risk factors that have been related to the development and prevalence of delirium during the ICU stay.12,33,34,36,38 Nevertheless, and always bearing in mind the multifactorial origin of ICU delirium, iatrogenic medication is considered a contributing and modifiable factor for this acute ICU brain dysfunction.54
The central cholinergic deficiency hypothesis is based on the increased risk of ICU delirium associated with the use of GABAA agonists and anticholinergic drugs.28 It has been proposed that the action of dexmedetomidine on the central α2 receptors (unlike benzodiazepines or propofol, which act on GABA receptors) is the key to the beneficial effects associated with its use in ICU patients with delirium.15,41•43 Besides, its anti-inflammatory effect may also contribute to reducing both the risk of delirium and the duration of the brain dysfunction, since inflammation appears to play an important role in the pathophysiology of delirium.55
GABAergic agents may induce delirium via a variety of mechanisms: by interrupting cholinergic muscarinic transmission at the level of the basal forebrain and hippocampus,56 increasing compensatory up-regulation of NMDA and Ca2+ channel activity,57 by disrupting thalamic pathways,58 causing withdrawal states after cessation, disrupting circadian rhythms of melatonin release59 and/or interfering with physiologic sleep patterns.60
Other medications that are typically administered to critically ill patients may also produce an imbalance in the neurotransmission of acetylcholine, dopamine, and GABA, thus affecting cortical and subcortical pathways involved in behavior, cognitive functioning, emotional regulation, and sleep. Anticholinergic drugs and their metabolites predominantly inhibit striatal cholinergic interneurons by blocking postsynaptic muscarinic receptors (especially M1), leading to hallucinations and attention deficit in post-operative patients.61 Tricyclic antidepressants, H2 blockers and opioids also have a central anticholinergic effect62 and possibly narcotics and paralytics as well.63 If the physiopathological mechanisms underlying the relation between sedative management and ICU delirium are unclear, even less is known about the way sedative and analgesic agents impact long-term cognition in ICU survivors.
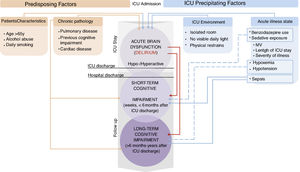
Finally, we must bear in mind the complexity and heterogeneity of the ICU patients who undergo MV. Various factors related to the illness itself and to its management can affect the functioning of the brain in these patients, and thus their cognitive states, in both the short and the long term. The etiology of the cognitive impairment in critically ill patients and in survivors must be regarded as multifactorial (Fig. 1). Hypoxemia events, dyspnea and air hunger, and even the MV itself are important components to consider. Experimental studies have shown that systemic PaO2 oscillations cause mild brain injury64; specifically, brain structures such as the hippocampus are extremely vulnerable to hypoxemia.65 Furthermore, dyspnea and air hunger cause activation of the afferent pathways through chemo or baroreceptors, reflected by an increase in the signal in magnetic resonance in brain areas such as the limbic system which are related to psychological, emotional and memory disorders.66 This neurocognitive compromise increases as a result of MV.67
Main predisposing and precipitating factors that critically ill patients may present at ICU admission and their relationship with the cognitive alterations during and after the ICU stay. Predisposing factors are considered as characteristics of patients or their chronic illness. Precipitating factors are related to the ICU environment and illness management, so they are considered more modifiable. Thus, precipitating factors may be potential targets for reducing the risk of cognitive impairment in ICU patients and survivors.
↧ Relation to. ↧ ↧ ↧ ↧ Possible relation to.
The physiological and mechanical mechanisms through which the injured lung and MV may lead to brain dysfunction have been reviewed elsewhere.68 Mechanoreceptors (baroreceptors/stretch receptors) or chemoreceptors located in the lung can be stimulated during MV, reaching the CNS by several pathways. Several experimental studies have described how MV can lead to brain alterations.69•71 The brain can respond to this information by altering the permeability of the blood•brain barrier, by modifying cerebral blood flow or even by causing neuronal alterations72,73 and neuroinflammation, which may generate memory dysfunction.74 Recent studies by our group have underlined the important role of patient-ventilator decoupling during MV.75,76 Asynchronies can be presented during the entire MV period77 and can be influenced by the level of sedation.78 The presence of asynchrony has been associated with poor outcomes such as longer duration of MV, greater incidence of tracheostomy, longer ICU stay and increased mortality.79,80 The relation of this patient-ventilator decoupling with sedation strategies and its impact on ICU acute brain dysfunction/delirium, and its neurocognitive sequelae in critically ill survivors, are issues that merit further study.
ConclusionWith the scarcity of the literature available, it would be premature to attempt to draw any firm conclusions on the impact of sedation during critical illness and its role in long-term cognitive deficit. The lack of studies designed for this purpose means that we cannot recommend particular types of sedative strategy during the ICU stay for improving cognitive status in critically ill patients at hospital discharge and during longer follow up. Nonetheless, this review of the current literature suggests that the different sedation strategies applied in ICU patients (daily sedation interruption, goal-directed sedation, or even no sedation) are not associated with a worse cognitive status in ICU survivors than usual treatment. In fact, the trend toward reducing sedation doses during the ICU stay may be related to better cognitive performance. Taking this into account, it seems that the management of sedation and analgesia in ICU patients may in some way be associated with cognitive status, and in particular with executive dysfunction, at hospital discharge and at short-term follow up.
The effect of the sedatives used during the ICU stay on long-term cognition has not yet been demonstrated. A clearer relationship has been described between the impact of ICU delirium and long-term cognitive impairment in ICU survivors. Moreover, sedatives such as benzodiazepines are known to increase the presence of delirium during critical illness. Thus, sedation strategy and management should aim to achieve an optimal condition and to reduce the prevalence of delirium during the ICU stay, in order to prevent long-term cognitive alterations.
Reducing levels of sedation during the ICU stay does not negatively impact the clinical outcomes of critically ill patients, and it improves certain aspects of their management and rehabilitation. Higher levels of awareness in patients allow fuller exploration of their cognitive status, pain, and dyspnea during critical illness and permit the application or improvement of different analgesic or management strategies. It facilitates patients tm) communication and collaboration with the clinical staff and favors active participation in their recovery process; it also allows them to interact with their family and friends, ensuring the emotional support needed during critical illness. Finally, other beneficial non-pharmacological interventions such as cognitive stimulation or early mobilization therapy can be applied as part of the rehabilitation process.
Funding supportThis review was carried out as part of the Neurocognition and Critically Ill Patients research line at the University Hospital Parc Taulí, which is co-funded by the Programs of Support to Research: SGR-1320 from the Agència de Gestió d tm)Ajuts Universitaris i de Recerca (AGAUR) Departament d tm)Empresa I Coneixement de la Generalitat de Catalunya, CIBER de Enfermedades Respiratorias from the Instituto de Salud Carlos III and Fundació Parc Taulí (Ref. CIR 2014/028). This project is part of the research programs PI13/02204 and PI16/01606 in the Spanish Plan Nacional de R+D+I and co-funded by the ISCIII-Subdirección General de Evaluación and Fondo Europeo de Desarrollo Regional (FEDER); Marató TV3 (ref. 112810) and CIBERES-CIBER BIOINGENIERIA BIOMATERIALES Y NANOMEDICINA (CIBER-BBN) (Ref. ES15PINT007).
Conflict of interestThe authors have no conflict of interest to disclose.
We thank the nurses and physicians from the Critical Care Unit of the Parc Taulí Hospital who participated either directly or indirectly in this review. Their answers to our queries have been indispensable. We also thank Michael Maudsley for reviewing and correcting the English manuscript.