The SARS-CoV-2 pandemic brought us a new plethora of patients with an extremely severe form of acute respiratory distress syndrome (ARDS).
Clinical autopsy can provide us with information to know and understand this new viral infection. The objective of this study is to characterize—at pulmonary level—the pathological anatomy (PA), microbiology, and viral load of a series of patients who died of SARS-CoV-2 related ARDS.
This was an observational prospective study of patients who had died at the intensive care unit (ICU) (teaching hospital; reference center for patients with SARS-CoV-2 infection) with a confirmed diagnosis of SARS-CoV-2 infection. The study was approved by the Spanish hospital ethics research committee (ASSE 2015). Informed consent from the closest family member was required.
Patients with ARDS (Berlin Definition)1 due to SARS-CoV-2 infection (December 2020 through November 2021) were included.
The main clinical characteristics are shown on Table 1 and the electronic supplementary data (ESD) Patients were categorized based on their ICU stay into 3 timeframes (up to 10 days, 11–20 days, over 20 days) based on the evolutionary stages of ARDS.2
General characteristics of the population (N = 21).
| Demographic characteristics | N = 21 |
| Age (years), mean (SD) | 62 ± 12 |
| Sex, feminine, n (%) | 6 (29) |
| Comorbidities | |
| COPD, n (%) | 8 (38) |
| AHT, n (%) | 10 (48) |
| Diabetes, n (%) | 4 (19) |
| Obesity, n (%) | 8 (38) |
| Variables on day 1 | |
| APACHE II, mean (SD) | 21 ± 9 |
| SOFA ESCORE, mean (SD) | 12 ± 3 |
| D-dimer (ng/mL), mean (SD) | 13,164 ± 17,718 |
| Ferritin (ng/mL), mean (SD) | 2448 ± 1329 |
| CRP (mg/L), mean (SD) | 175 ± 89 |
| LDH (U/L), mean (SD) | 849 ± 432 |
| Lymphocytes/mm 3, mean (SD) | 631 ± 427 |
| Organ dysfunctions | |
| Circulatory shock, n (%) | 17 (85) |
| AKI, n (%) | 11 (55) |
| Cause of death | |
| Multiple organ failure, n (%) | 4 (19) |
| Refractory hypoxemia, n (%) | 9 (43) |
| Circulatory shock, n (%) | 8 (38) |
| Days on mechanical ventilation, mean (SD) | 15 ± 10 |
| ICU stay (days), mean (SD) | 17 ± 10 |
AHT, arterial hypertension; AKI, acute kidney injury; APACHE II, Acute Physiology and Chronic Health Evaluation II; COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; LDH, lactate dehydrogenase; SOFA, Sequential Organ Failure Assessment.
Autopsies were performed 3 h prior to death to preserve the quality of microbiological samples. A macroscopic study of the lung was conducted and samples from the most compromised areas were drawn including microbiological (Gram staining, cultures, and mycological study), and histological analyses (hematoxylin/eosin staining) using Masson’s Trichome Staining to rule out fibrosis and thrombosis. Viral analysis through RNA extraction, and viral genome quantification (RT-PCR technique) were conducted as well. In addition, a Cycle Threshold (Ct) < 35 was defined as a positive.3
A total of 21 patients were included. Macroscopy revealed the presence of heavy edematous (1130 ± 349 g), hemorrhagic, and erythro-cyanotic lungs (ESD).
Microscopy revealed the presence of diffuse alveolar damage (DAD) in 20 cases (95%) (ESD).
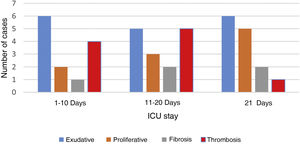
In patients dead until day #10 (ICU stay), and in those dead between day #11 and day #20, the exudative stage was predominant and coexisted with proliferative elements and early fibrosis. In those patients who stayed at the ICU over 20 days, the proliferative pattern was predominant. Still exudative elements persisted being this pattern predominant in 2 cases (Fig. 1).
Pulmonary microvascular thrombosis was confirmed in 10 patients and predominant in those with ICU stays < 20 days (Fig. 1).
Lung tissue cultures tested positive in 17 cases (81%). In 6 cases (85%) cultures tested positive in patients with ICU stays up to 10 days. In another 6 (75%) cultures tested positive in patients with ICU stays between 11 and 20 days, and in 5 cases (83%) cultures tested positive in patients with ICU stays over 20 days. In the former, sensitive germs were found. However, after 20 days at the ICU, candida and extended-resistance and pandrug-resistant germs were isolated.4 Aspergillus was isolated in 2 cases (also seen in the AP examination) that turned out to be 2 cases of invasive pulmonary Aspergillosis.5
The SARS-CoV-2 viral load was analyzed in 17 patients. The samples showed high viral loads up to 30 days after symptom onset (ESD).
Anatomopathological findings revealed the presence of predominant DAD similar to what has been reported in patients with ARDS without SARS-CoV-2 infection. This is consistent with the findings made by Katzenstein et al.2 who revealed a predominant exudative pattern within the first 10 days followed by proliferative elements and fibrosis from week #2.
However, the presence of exudative elements was the predominant pattern in some cases after 3 weeks of ICU stay, which is different compared to Katzenstein et al.’s findings2 that limit this exudative stage within the first 15 days. This is consistent with the findings made by Thille who found that 17% of lung autopsies had exudative patterns after 3 weeks. Similarly, cases with predominant proliferative changes within the first week of disease progression were found. This is consistent with the fact that proliferative stage can have a very early start.6
Another anatomopathological aspect we should mention is the frequency of pulmonary microthrombosis found in 50% of the cases. This has already been described in dead patients with SARS-CoV-2 infections.7 It is a phenomenon known as ARDS2,8 that can be observed early in the DAD setting, and is consistent with our findings where thrombosis was an early finding. Recently, Ackermann confirmed the presence of precapillary microthrombosis in patients with SARS-CoV-2 and H1N1 co-infection. However, the compromise of microthrombosis at the pulmonary capillary networks was 9 times higher in cases of SARS-CoV-2.7 This difference can explain the high degree of severe and refractory hypoxemia seen. Refractory hypoxemia amounts to 15% of all deaths due to non-SARS-CoV-2 induced ARDS8 while, in our series, it was directly responsible for 43% of all deaths reported.
A high percentage of positive cultures in patients with ICU stays of up to 10 days and resistant germs being isolated (consistent with the unit’s ecology) was reported in the microbiological findings made in patients with prolonged ICU stays. The clinical significance of candida isolation in this group of patients is still pending assessment.
The viral load found in the lung tissue revealed high and persistent levels. This is consistent with what Schurink et al. described.9 However, it is different from the findings made by Merdji et al. who found no viral remains after the first week in lung samples obtained through biopsic punctures, which may explain the difference reported.10
Our study has some limitations. In the first place, it was conducted in only single center and the number of patients included was small. The strengths of the study were how fast autopsies were performed, which allowed microbiological examinations and analyses of the viral load found in lung tissues.
In conclusion, ARDS due to SARS-CoV-2 has an anatomopathological pattern characterized by DAD, which is similar to ARDS due to different etiologies. We confirmed the presence of a pattern with changes based on the time of evolution, although both the persistence and predominance of the exudative pattern in cases with > 30 days of evolution were significant. We should also mention the high prevalence of microthrombosis and how persistent the SARS-CoV-2 virus is in the lung parenchyma with high viral loads even in patients with prolonged disease.