To compare outcomes between intensivist-directed and cardiac surgeon-directed care delivery models.
DesignThis retrospective, historical-control study was performed in a cohort of adult cardiac surgical patients at Zhongshan Hospital (Fudan University, China). During the first phase (March to August 2015), cardiac surgeons were in charge of postoperative care while intensivists were in charge during the second phase (September 2015–June 2016). Both phases were compared regarding successful extubation rate, intensive care unit (ICU) length of stay (LOS), and in-hospital mortality.
SettingTertiary Zhongshan Hospital (Fudan University, China).
PatientsConsecutive adult patients admitted to the cardiac surgical ICU (CSICU) after heart surgery.
InterventionsPhase I patients treated by cardiac surgeons, and phase II patients treated by intensivists.
Main variables of interestSuccessful extubation, ICU LOS and in-hospital mortality.
ResultsA total of 1792 (phase I) and 3007 patients (phase II) were enrolled. Most variables did not differ significantly between the two phases. However, patients in phase II had a higher successful extubation rate (99.17% vs. 98.55%; p=0.043) and a shorter median duration of mechanical ventilation (MV) (18 vs. 19h; p<0.001). In relation to patients with MV duration >48h, those in phase II had a comparatively higher successful extubation rate (p=0.033), shorter ICU LOS (p=0.038) and a significant decrease in in-hospital mortality (p=0.039).
ConclusionsThe intensivist-directed care model showed improved rates of successful extubation and shorter MV durations after cardiac surgery.
Comparar el manejo entre intensivistas y cirujanos de pacientes de cirugía cardíaca en la unidad de cuidados intensivos.
DiseñoEste estudio de control retrospectivo se llevó a cabo con una cohorte de pacientes adultos de cirugía cardíaca. Durante la primera fase (de marzo a agosto de 2015), los cirujanos cardíacos estuvieron a cargo del manejo médico en la unidad; y durante la segunda fase (septiembre de 2015 a junio de 2016), lo hicieron intensivistas. Comparamos las fases en cuanto al número de extubaciones exitosas, el tiempo de estancia y la mortalidad.
Lugar del estudioHospital Zhongshan de la Universidad de Fudan, China.
PacientesAdultos admitidos secuencialmente a la unidad de cuidados intensivos cardíacos después de intervenciones quirúrgicas.
IntervencionesManejo médico por cirujanos en la primera fase, y por intensivistas en la segunda fase.
Variables prioritariasExtubación exitosa, tiempo de estancia en la unidad de cuidados intensivos y mortalidad.
ResultadosParticiparon 1.792 pacientes en la fase i y 3.007 en la fase ii. Los pacientes de la fase ii tuvieron más extubaciones exitosas (99,17 frente al 98,55%, p=0,043), y necesitaron menos tiempo de ventilación mecánica (mediana de 18 frente a 19h, p<0,001). De entre los pacientes con ventilación mecánica de más de 48h, los de la fase iifueron extubados exitosamente más veces, tuvieron una estancia más corta (p=0,038), y una menor mortalidad (p=0,039).
ConclusionesEl manejo médico por intensivistas aumentó significativamente el número de extubaciones exitosas y disminuyó el tiempo de ventilación mecánica.
Cardiac surgery is now frequently offered to patients with advanced age, diabetes mellitus, chronic obstructive pulmonary disease, higher European system for cardiac operative risk evaluation II (EuroSCORE II) scores, and lower left ventricular ejection fractions, etc. Thus, the comorbidities in cardiac surgical patients have made the cases more complex over the past two decades.1–4 This demographic change has resulted in a higher incidence of perioperative complications, prolonged intensive care unit (ICU) and hospital lengths of stay (LOS), and even hospital mortalities.5–9 As a result, the management of cardiac surgical patients requires a new level of critical care performance with introduction of dedicated and specialized critical care physicians.
Although the American Heart Association has recommended modifications to the staffing model for cardiac patients’ care units,10 substantial variability remains in the organization for cardiac surgical intensive care units (CSICU).11 In China, most CSICUs are affiliated to the department of cardiac surgery and are directed by cardiac surgeons. But the intensivists may have a role in many aspects of patient care, particularly in improving ventilatory support, and the role of cardiac surgeons in CSICUs may be reduced in the future. Studies have evaluated the role of intensivists on patient outcomes after cardiac surgery, but have focused on mortality and ICU LOS.12–14 Few studies have paid attention to the ratio of successful extubations and no one has tried to demonstrate the reasons for extubation failure in adult cardiac surgical patients. The aim of this study was to assess whether an intensivist-directed care delivery model could improve the ratio of successful extubation after cardiac surgery and decrease ICU LOS and in-hospital mortality.
Patients and methodsStudy designThis single-center, retrospective, historical-control study was performed in a cohort of adult patients who underwent cardiac surgery at the Zhongshan Hospital of the Fudan University from March 2015 to June 2016. This is an academic teaching hospital with more than 3000 cardiac surgical procedures per year. In September 2015, the management of CSICU in our hospital was transferred from the department of cardiac surgery to the department of critical care medicine for quality improvement. Thus, a different care delivery model directed by intensivists was adopted. Accordingly, we collected clinical data of patients in the CSICU from March to August 2015 for a phase I group and from September 2015 to June 2016 for a phase II group. In phase I, cardiac surgeons who spent most of their time in the operating room directed the care delivery. Therapy decisions including extubation and discharges from the CSICU were often made before they got into the operation room. The cardiac surgeons managed the patients principally according to their personal experience, with consultant physicians from other departments including radiology, ultrasonography, and respiratory departments. In phase II, intensivists directed the care delivery and were 24h/7 days on duty in the CSICU. Physicians from other departments supported the intensivists and specialized techniques (point of care [POC] ultrasound, fiberoptic scope, and advanced hemodynamic monitoring) were available to them. All intensivists extubated the patients according to the same criteria: clear consciousness, stable hemodynamics, adequate oxygenation, and successful spontaneous breathing trial. The spontaneous breathing trial was carried out using a continuous positive airway pressure or pressure support model, with pressure support at 5cm H2O and positive end expiratory pressures at 5cm H2O, lasting 30–60min. The patients passed the spontaneous breathing trial if none of the following criteria were present: breathing frequency >35breaths/min, SpO2 <90%, rapid shallow breathing index (respiratory rate/tidal volume) >105 breaths/min/L, 20% increase or decrease from the baseline heart rate or blood pressure, use of accessory muscles, abdominal paradox movement, substantial agitation, anxiety, and/or diaphoresis.15,16 If the patient was not able to pass the spontaneous breathing trial, the intensivists tried to detect the reason for the failure using disciplinary related practices. The intensivists followed the directive protocol strictly regarding the timing of patient transferring to the ward: (1) weaning from various life support techniques; (2) respiratory and hemodynamic stabilities; (3) lack of severe discomfort feelings.
The primary outcome of this study was the improvement in successful extubations defined as not requiring reintubation within the first 48h after the extubation. The secondary outcomes were the improvement of in-hospital mortality and the reduced ICU LOS.
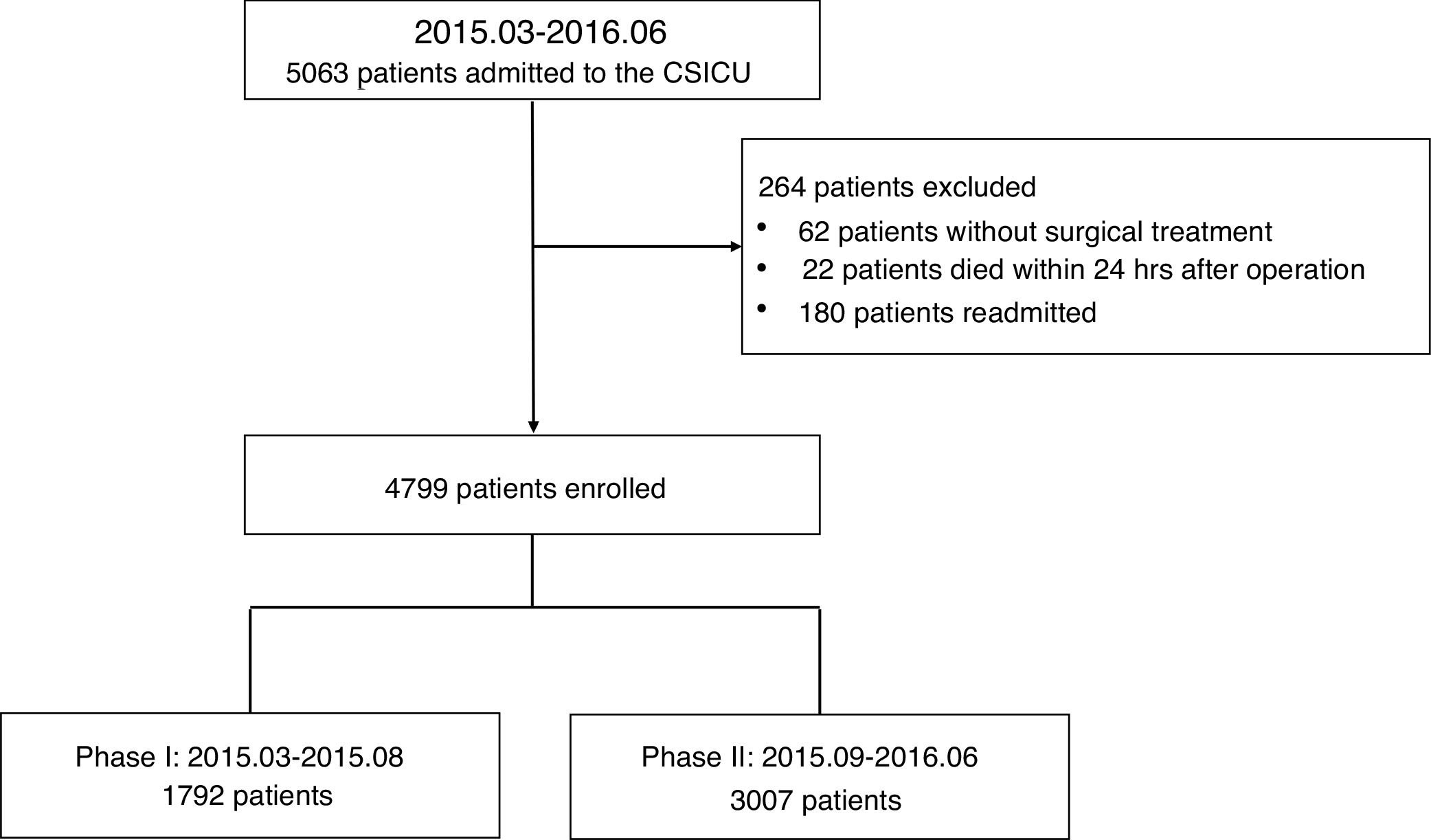
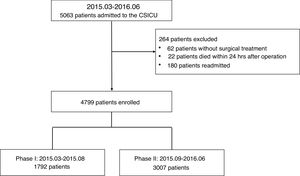
PopulationAll consecutive patients older than 18 years, who underwent cardiac surgery between March 2015 and June 2016, were enrolled in this study. We excluded patients who did not undergo surgical treatment, those who died within 24h after surgery, and those readmitted to the CSICU. The Ethical Committee of Zhongshan hospital affiliated to the Fudan University approved this study (NO. B2018-011). Informed consent forms were not required because this retrospective study did not modify existing diagnostic or therapeutic strategies.
DefinitionsExtubation failure was asserted in cases needing reintubation within 48h after extubation. Physicians identified atelectasis and pulmonary edema based on clinical manifestations, chest radiographies and/or ultrasound examinations. Stroke was defined as brain hemorrhage or infarction confirmed by radiographic examinations. CO2 retention was identified in cases with high arterial blood CO2 partial pressure and lethargy. The MV duration was defined as the time between ICU admission and the first extubation.
Data collectionThe following data were recorded:
- (1)
Baseline demographic data (age, gender, body mass index).
- (2)
Patients’ degree of disease severity (Acute Physiology and Chronic Health Evaluation II [APACHE II] score and EuroSCORE).
- (3)
Type of surgery (valve, coronary artery bypass graft [CABG], valve and CABG, aortic, congenital and others).
- (4)
Postoperative characteristics (renal replacement therapy [RRT], intra-aortic balloon pump [IABP], extracorporeal membrane oxygenation [ECMO], POC ultrasound, fiberoptic scope examination, computed tomography [CT] scan, and Pulse Contour Cardiac Output monitoring system [PiCCO]).
- (5)
Outcome (MV duration, tracheotomy, noninvasive ventilation, successful extubation, ICU LOS, readmission, and in-hospital mortality).
Continuous variables were presented as means±SD or medians (25%–75% interquartile ranges, IQRs), while categorical variables were reported as adjusted proportions. Continuous data were compared using student t-test or the Mann–Whitney U test as appropriate, while differences between categorical variables were compared using the chi-square test or Fisher's exact test when necessary. A p value <0.05 was considered statistically significant. Statistical analyses were performed using the stata13.0 and SPSS 22.0 software (IBM Corporation, NY, USA).
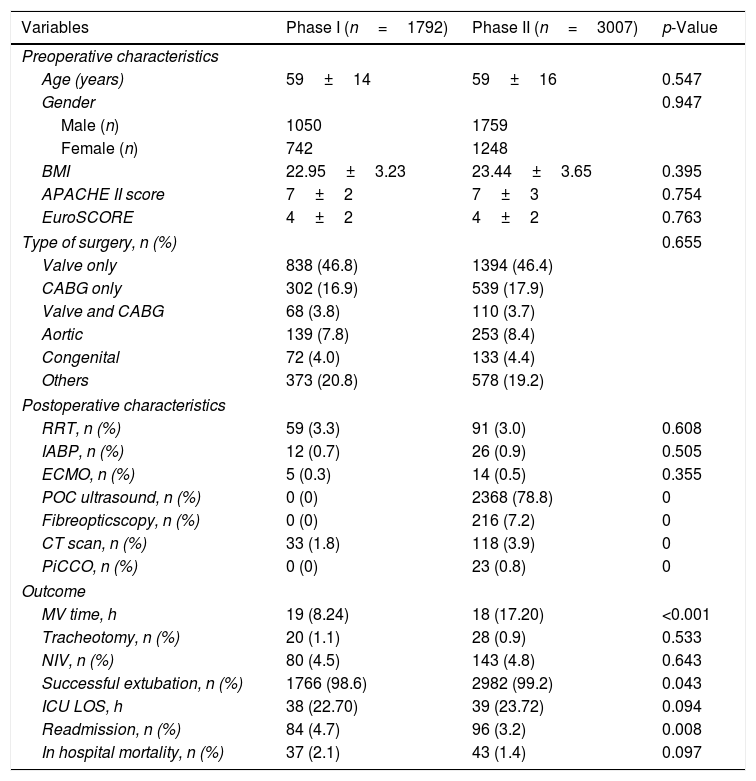
ResultsDemographic and clinical characteristics of enrolled patientsA total of 4799 patients were enrolled in this study. A patient flowchart was shown in Fig. 1. The patients enrolled in the phase I and phase II groups were 1792 and 3007, respectively. Demographic characteristics, APACHE II score, EuroSCORE, surgery type, postoperative characteristics, and outcomes were shown in Table 1. We found no significant differences between the two phases in terms of age, APACHE II score, EuroSCORE or type of surgery (5 subgroups: valve only, CABG only, valve and CABG, aortic, congenital and others). The cardiac surgeons did not perform POC ultrasound and fiberoptic scope examinations themselves, nor did they perform advanced hemodynamic monitoring. In phase I, the cardiac surgeons took 33 out of 1792 (1.8%) patients for CT scans; while in phase II, the intensivists took 118 out of 3007 (3.9%) patients for CT scans.
Characteristics of patients in the two phase-groups.
| Variables | Phase I (n=1792) | Phase II (n=3007) | p-Value |
|---|---|---|---|
| Preoperative characteristics | |||
| Age (years) | 59±14 | 59±16 | 0.547 |
| Gender | 0.947 | ||
| Male (n) | 1050 | 1759 | |
| Female (n) | 742 | 1248 | |
| BMI | 22.95±3.23 | 23.44±3.65 | 0.395 |
| APACHE II score | 7±2 | 7±3 | 0.754 |
| EuroSCORE | 4±2 | 4±2 | 0.763 |
| Type of surgery, n (%) | 0.655 | ||
| Valve only | 838 (46.8) | 1394 (46.4) | |
| CABG only | 302 (16.9) | 539 (17.9) | |
| Valve and CABG | 68 (3.8) | 110 (3.7) | |
| Aortic | 139 (7.8) | 253 (8.4) | |
| Congenital | 72 (4.0) | 133 (4.4) | |
| Others | 373 (20.8) | 578 (19.2) | |
| Postoperative characteristics | |||
| RRT, n (%) | 59 (3.3) | 91 (3.0) | 0.608 |
| IABP, n (%) | 12 (0.7) | 26 (0.9) | 0.505 |
| ECMO, n (%) | 5 (0.3) | 14 (0.5) | 0.355 |
| POC ultrasound, n (%) | 0 (0) | 2368 (78.8) | 0 |
| Fibreopticscopy, n (%) | 0 (0) | 216 (7.2) | 0 |
| CT scan, n (%) | 33 (1.8) | 118 (3.9) | 0 |
| PiCCO, n (%) | 0 (0) | 23 (0.8) | 0 |
| Outcome | |||
| MV time, h | 19 (8.24) | 18 (17.20) | <0.001 |
| Tracheotomy, n (%) | 20 (1.1) | 28 (0.9) | 0.533 |
| NIV, n (%) | 80 (4.5) | 143 (4.8) | 0.643 |
| Successful extubation, n (%) | 1766 (98.6) | 2982 (99.2) | 0.043 |
| ICU LOS, h | 38 (22.70) | 39 (23.72) | 0.094 |
| Readmission, n (%) | 84 (4.7) | 96 (3.2) | 0.008 |
| In hospital mortality, n (%) | 37 (2.1) | 43 (1.4) | 0.097 |
Abbreviations: BMI, body mass index; APACHE, Acute Physiology and Chronic Health Evaluation; EuroSCORE, European system for cardiac operative risk evaluation; CABG, coronary artery bypass graft; RRT, renal replacement therapy; IABP, intra-aortic balloon pump; ECMO, extracorporeal membrane oxygenation; POC, point of care; CT, computed tomography; PiCCO, Pulse Contour Cardiac Output monitoring system; MV, mechanical ventilation; NIV, non-invasive ventilation; LOS, length of stay.
In phase II, patients had a higher ratio of successful extubation (99.2%) than in phase I (98.6%), p=0.043, together with a shorter median MV time (18 vs. 19h, p<0.001). The differences in in-hospital mortality between the phase II (1.4%) and phase I (2.1%) and ICU LOS (39 vs. 38h) were not significant. However, the readmission rate in phase II was lower (3.2%) than in phase I (4.7%), p=0.008. ECMO, RRT or IABP therapies were occasionally used in both phases. Tracheotomy (0.9% vs. 1.1%) and noninvasive ventilation (4.8% vs. 4.5%) were comparable in the two phases as well (Table 1).
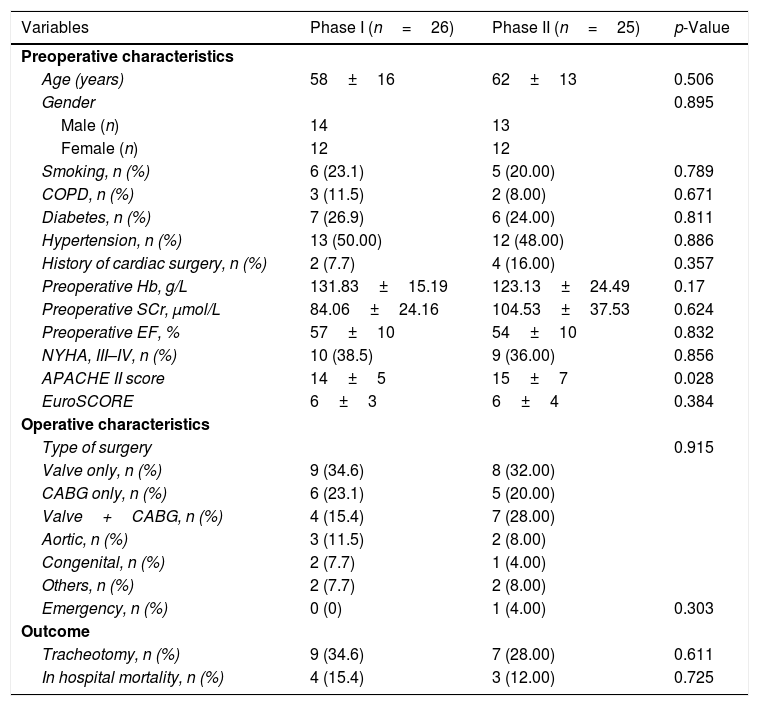
Characteristics of the patients with extubation failureIn phase I, 26 out of 1792 patients were reintubated within 48h after extubation, and in phase II, 25 out of 3007 patients were reintubated within 48h after extubation. Demographic characteristics were comparable between the two phases. There were also no significant differences in terms of the type of surgery, tracheotomy and mortality between the two phases (Table 2). We found significant differences between the two phases concerning the causes of extubation failure (Table 3).
Characteristics of patients with extubation failure.
| Variables | Phase I (n=26) | Phase II (n=25) | p-Value |
|---|---|---|---|
| Preoperative characteristics | |||
| Age (years) | 58±16 | 62±13 | 0.506 |
| Gender | 0.895 | ||
| Male (n) | 14 | 13 | |
| Female (n) | 12 | 12 | |
| Smoking, n (%) | 6 (23.1) | 5 (20.00) | 0.789 |
| COPD, n (%) | 3 (11.5) | 2 (8.00) | 0.671 |
| Diabetes, n (%) | 7 (26.9) | 6 (24.00) | 0.811 |
| Hypertension, n (%) | 13 (50.00) | 12 (48.00) | 0.886 |
| History of cardiac surgery, n (%) | 2 (7.7) | 4 (16.00) | 0.357 |
| Preoperative Hb, g/L | 131.83±15.19 | 123.13±24.49 | 0.17 |
| Preoperative SCr, μmol/L | 84.06±24.16 | 104.53±37.53 | 0.624 |
| Preoperative EF, % | 57±10 | 54±10 | 0.832 |
| NYHA, III–IV, n (%) | 10 (38.5) | 9 (36.00) | 0.856 |
| APACHE II score | 14±5 | 15±7 | 0.028 |
| EuroSCORE | 6±3 | 6±4 | 0.384 |
| Operative characteristics | |||
| Type of surgery | 0.915 | ||
| Valve only, n (%) | 9 (34.6) | 8 (32.00) | |
| CABG only, n (%) | 6 (23.1) | 5 (20.00) | |
| Valve+CABG, n (%) | 4 (15.4) | 7 (28.00) | |
| Aortic, n (%) | 3 (11.5) | 2 (8.00) | |
| Congenital, n (%) | 2 (7.7) | 1 (4.00) | |
| Others, n (%) | 2 (7.7) | 2 (8.00) | |
| Emergency, n (%) | 0 (0) | 1 (4.00) | 0.303 |
| Outcome | |||
| Tracheotomy, n (%) | 9 (34.6) | 7 (28.00) | 0.611 |
| In hospital mortality, n (%) | 4 (15.4) | 3 (12.00) | 0.725 |
Abbreviations: COPD, chronic obstructive pulmonary disease; Hb, hemoglobin; SCr, serum creatinine; EF, ejection fraction; NYHA, New York Heart Association; APACHE, Acute Physiology and Chronic Health Evaluation; EuroSCORE, European system for cardiac operative risk evaluation; CABG, coronary artery bypass graft.
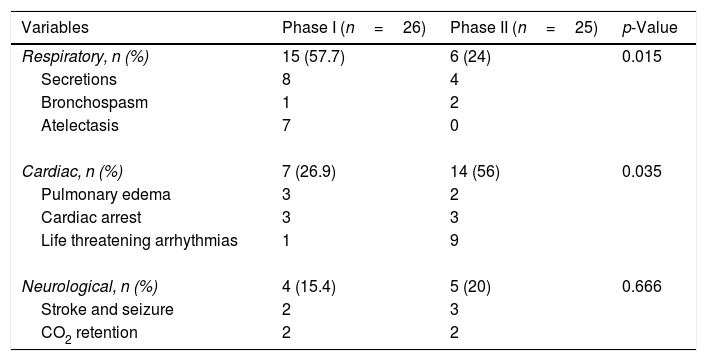
Reasons of extubation failure.
| Variables | Phase I (n=26) | Phase II (n=25) | p-Value |
|---|---|---|---|
| Respiratory, n (%) | 15 (57.7) | 6 (24) | 0.015 |
| Secretions | 8 | 4 | |
| Bronchospasm | 1 | 2 | |
| Atelectasis | 7 | 0 | |
| Cardiac, n (%) | 7 (26.9) | 14 (56) | 0.035 |
| Pulmonary edema | 3 | 2 | |
| Cardiac arrest | 3 | 3 | |
| Life threatening arrhythmias | 1 | 9 | |
| Neurological, n (%) | 4 (15.4) | 5 (20) | 0.666 |
| Stroke and seizure | 2 | 3 | |
| CO2 retention | 2 | 2 | |
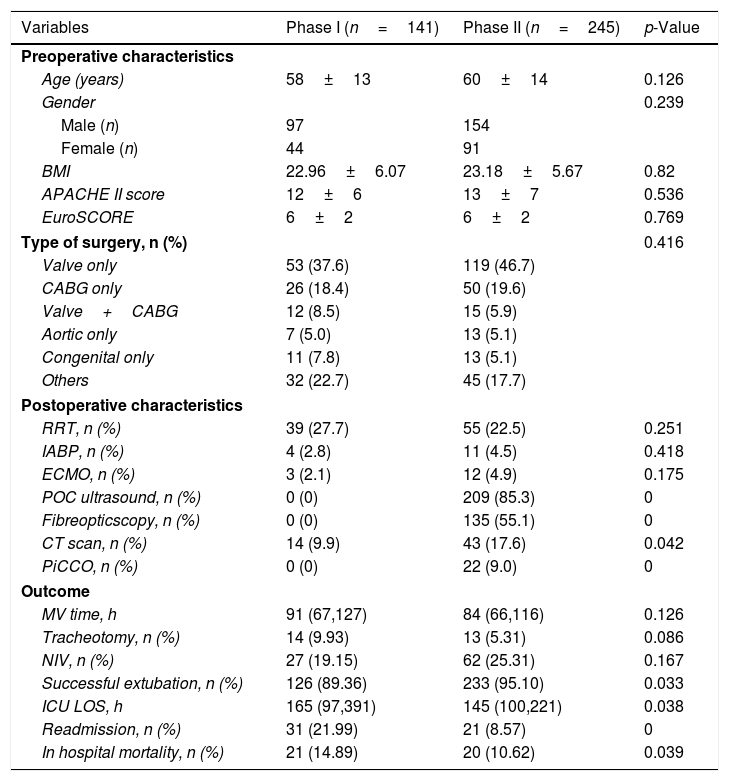
In phase I, 141 out of 1792 patients (7.9%) received MV for more than 48h whilst in phase II, 245 out of 3007 patients (8.1%) underwent MV for longer than 48h. We found no significant differences in the two phases in terms of age, APACHE II, EuroSCORE and type of surgery. The two phases had patients undergoing similar life support techniques such as RRT, IABP and ECMO. In phase II, the intensivists performed more POC ultrasound and fiberoptic scope examinations, and they also took more patients for CT scans. We found no significant differences between the two phases in terms of MV durations, NIV or tracheotomy. Compared with phase I, phase II had a significantly higher ratio of successful extubations, decreasing in-hospital mortality, readmission rate, and lower ICU LOS (Table 4).
Characteristics of the patients with MV duration over 48h.
| Variables | Phase I (n=141) | Phase II (n=245) | p-Value |
|---|---|---|---|
| Preoperative characteristics | |||
| Age (years) | 58±13 | 60±14 | 0.126 |
| Gender | 0.239 | ||
| Male (n) | 97 | 154 | |
| Female (n) | 44 | 91 | |
| BMI | 22.96±6.07 | 23.18±5.67 | 0.82 |
| APACHE II score | 12±6 | 13±7 | 0.536 |
| EuroSCORE | 6±2 | 6±2 | 0.769 |
| Type of surgery, n (%) | 0.416 | ||
| Valve only | 53 (37.6) | 119 (46.7) | |
| CABG only | 26 (18.4) | 50 (19.6) | |
| Valve+CABG | 12 (8.5) | 15 (5.9) | |
| Aortic only | 7 (5.0) | 13 (5.1) | |
| Congenital only | 11 (7.8) | 13 (5.1) | |
| Others | 32 (22.7) | 45 (17.7) | |
| Postoperative characteristics | |||
| RRT, n (%) | 39 (27.7) | 55 (22.5) | 0.251 |
| IABP, n (%) | 4 (2.8) | 11 (4.5) | 0.418 |
| ECMO, n (%) | 3 (2.1) | 12 (4.9) | 0.175 |
| POC ultrasound, n (%) | 0 (0) | 209 (85.3) | 0 |
| Fibreopticscopy, n (%) | 0 (0) | 135 (55.1) | 0 |
| CT scan, n (%) | 14 (9.9) | 43 (17.6) | 0.042 |
| PiCCO, n (%) | 0 (0) | 22 (9.0) | 0 |
| Outcome | |||
| MV time, h | 91 (67,127) | 84 (66,116) | 0.126 |
| Tracheotomy, n (%) | 14 (9.93) | 13 (5.31) | 0.086 |
| NIV, n (%) | 27 (19.15) | 62 (25.31) | 0.167 |
| Successful extubation, n (%) | 126 (89.36) | 233 (95.10) | 0.033 |
| ICU LOS, h | 165 (97,391) | 145 (100,221) | 0.038 |
| Readmission, n (%) | 31 (21.99) | 21 (8.57) | 0 |
| In hospital mortality, n (%) | 21 (14.89) | 20 (10.62) | 0.039 |
Abbreviations: BMI, body mass index; APACHE, Acute Physiology and Chronic Health Evaluation; EuroSCORE, European system for cardiac operative risk evaluation; CABG, coronary artery bypass graft; RRT, renal replacement therapy; IABP, intra-aortic balloon pump; ECMO, extracorporeal membrane oxygenation; POC, point of care; CT, computed tomography; PiCCO, Pulse Contour Cardiac Output monitoring system; MV, mechanical ventilation; NIV, non-invasive ventilation; LOS, length of stay.
In this historical cohort study comparing two different care delivery models in our dedicated CSICU, we found a significant improvement in the ratio of successful extubations in the intensivist-directed care delivery model. Additionally, the MV duration was shorter compared with that in the cardiac surgeon-directed model. The main reason for extubation failure in the phase I group was the presence of adverse respiratory variables such as secretions and atelectasis, while in phase II it was the presence of adverse cardiac variables. Other important clinical outcomes such as ICU LOS and in-hospital mortality were similar between the two groups. However, for the patients whose MV lasted >48h, the ICU LOS and in-hospital mortality were both significantly better in the intensivist-directed care delivery model.
The rate of successful extubations is an important index to evaluate the quality of an ICU. This is especially true in the CSICU because almost all the cardiac surgical patients are admitted with mechanical ventilation postoperatively.17–19 To successfully extubate a patient, the physicians need to assess not only the pulmonary function but also other organ performances. The main training processes for intensivists and cardiac surgeons are quite different. The intensivists focus mainly on organ support techniques, while the cardiac surgeons care more about surgical related issues. Aging populations present more complicated situations and cardiothoracic critical care has become a new subspecialty requiring a specific curriculum and ICU experience, which is not a specialty of surgical trainees.20–22 Intensivists perform a more thorough systematic evaluation of the patients and, thus, monitor important variables affecting successful extubation. Studies have shown that systematically evaluating risk factors other than pulmonary mechanics may reduce extubation failure.23 To the best of our knowledge, this is the first study to assess the influence of different care delivery models on the ratio of successful extubation in adult cardiac surgical patients.
In this study, the intensivist-directed care delivery model not only improved the rate of successful extubations but also reduced the MV duration. This may be attributed to the different care delivery models. Although the reason for shorter mechanical ventilation periods during the phase II remained undetermined, our findings were consistent with those of previous studies.24–26 We hypothesize that perhaps it was the intensivists’ presence at the bedside being able to diagnose and treat patients more quickly. And, we suggest that the intensivist-directed care delivery model may facilitate earlier extubations. Patients in phase II received more advanced hemodynamic monitoring and POC examinations, such as bedside ultrasound examination. POC ultrasound can help to quickly identify the causes of weaning failure such as pulmonary edema,27 pneumothorax, pulmonary atelectasis, and even diaphragmatic dysfunction.28 And, advanced hemodynamical monitoring such as PiCCO also helps in guiding fluid resuscitation, and may lead to earlier extubations in hemodynamic compromised patients.29
The differences of other major clinical outcomes such as in-hospital mortality and ICU LOS were not significant between phase I and phase II patients. This is consistent with studies regarding the influence of transition to a 24/7 in-house intensivist care delivery model on postoperative outcomes.13,26 However, other studies have shown that staffing ICUs with intensivists may improve clinical outcomes such as mortality and ICU LOS.30–32 The mortality and ICU LOS are influenced by many factors, including the population enrolled. More than 90% of our patients were extubated within 48h after the operation. This group of patients displayed very low mortality and were often discharged within 24h after extubation, so their short ICU stay may not have provided enough time to show differences or benefits associated with the type of care received. Additionally, the shorter median MV duration in phase I among all patients was not clinically meaningful; and therefore, we reanalyzed our data in patients whose MV lasted >48h after the operation, and found that reintubations, mortality and ICU LOS were significantly reduced in those in the phase II. Additionally, our data showed that patients with prolonged mechanical ventilation had higher APACHE II scores, and higher frequencies of IABP, RRT, PiCCO or ECMO usage. The predictors of prolonged mechanical ventilation after cardiac surgery include older age, cardiac dysfunction, chronic renal failure, chronic obstructive pulmonary disease, repeated surgery, emergency surgery, higher New York Heart Association/Canadian Cardiovascular Society class, longer cardiopulmonary bypass time, blood product transfusions,33 early postoperative hemodynamic status, and events such as stroke and bacteremia.34 In this study, the possible causes for prolonged mechanical ventilation were the presence of underlying chronic diseases before surgery, hemodynamic instability and surgery related complications. Management of these patients often required a broad view of both cardiac and other organ functions. The more comprehensive management offered by the intensivists may have resulted in the improved outcomes of the patients with prolonged mechanical ventilation in the second phase. Collectively, our results indicate that patients with prolonged mechanical ventilation may get more benefits from the intensivist-directed care delivery model.
The extubation failure may cause increased morbidity and longer ICU LOS.35,36 The main causes of extubation failure include respiratory, cardiac and neurological variables, with respiratory failure being the most common cause.37–39 However, Forouzan et al. demonstrated that cardiac variables prompt more reintubations after cardiac surgery in adult patients than the respiratory variables.40 We found significant differences on the causes of extubation failure between the two phase-groups. In phase I, 15 out of the 26 extubation failure cases (57.7%) were reintubated for respiratory reasons. But, respiratory variables such as secretions, bronchospasm, and atelectasis are reversible if managed properly. In phase II, only 6 out of the 25 (24%) extubation failure cases were reintubated for respiratory reasons. The majority of the reintubations were due to life-threatening arrhythmias and cardiac arrest, which typically cannot be prevented and are less amenable to treatment. We propose that intensivists are more skillful in airway management. Intensivists addressed most of the respiratory causes for extubation failure, and thus cardiac variables (56%) became the main cause of extubation failure. Our results indirectly indicate that the intensivist-directed care delivery model in our CSICU was superior to the cardiac surgeon-directed model in terms of patient extubation management.
We are aware of the limitations of our study. First, this was a retrospective study, so we could not collect and analyze all clinical data details, such as results of POC ultrasound, hemodynamic monitoring and fiberoptic scope examinations. Second, whether the difference in care delivery models and the improved outcomes (reduced MV duration and higher ratio of successful extubation) had a cause-effect relationship remains to be confirmed. Finally, this was a single center study, and the results may not be applicable to other ICUs. Further prospective, multicenter trials are necessary to confirm the conclusions of this study.
ConclusionsCompared with the cardiac surgeon-directed care delivery model, the intensivist-directed model led to an improved successful extubation rate as well as a shorter duration of MV after cardiac surgery. The intensivist-directed care delivery model is superior and feasible in our CSICU, and should be considered for other CSICUs. Further research will be required before more definitive recommendations can be made.
Author's contributionsGuang-Wei Hao, Guo-Guang Ma and Bo-Fei Liu performed the literature search, extracted the data and drafted the manuscript. Xiao-Mei Yang, Ying Zhang, Lan Liu, Hua Liu and Ya-Min Zhuang reviewed studies for inclusion and extracted data. Guo-Wei Tu, Du-Ming Zhu and Zhe Luo performed the analysis and helped draft the manuscript. Guo-Wei Tu and Zhe Luo conceived the idea, participated in manuscript writing and revision. All authors have read and approved the final manuscript.
FundingThis article was supported by grants from the Natural Science Foundation of Shanghai (16ZR1405600), the National Natural Science Foundation of China (81500067), the Health and Family Planning Commission of Shanghai (20154Y011) and research funds of from the Zhong Shan Hospital (2017ZSYXQN23 and 2017ZSQN16).
Conflicts of interestThe authors declare that they have no conflicts of interest.