Given the importance of the management of sedation, analgesia and delirium in Intensive Care Units, and in order to update the previously published guidelines, a new clinical practice guide is presented, addressing the most relevant management and intervention aspects based on the recent literature. A group of 24 intensivists from 9 countries of the Pan-American and Iberian Federation of Societies of Critical Medicine and Intensive Therapy met to develop the guidelines. Assessment of evidence quality and recommendations was made according to the Grading of Recommendations Assessment, Development and Evaluation Working Group. A systematic search of the literature was carried out using MEDLINE, Cochrane Library databases such as the Cochrane Database of Systematic Reviews (CDSR) and the Cochrane Central Register of Controlled Trials (CENTRAL), the Database of Abstracts of Reviews of Effects (DARE), the National Health Service Economic Evaluation Database (NHS EED) and the database of Latin American and Caribbean Literature in Health Sciences (LILACS). A total of 438 references were selected. After consensus, 47 strong recommendations with high and moderate quality evidence, 14 conditional recommendations with moderate quality evidence, and 65 conditional recommendations with low quality evidence were established. Finally, the importance of initial and multimodal pain management was underscored. Emphasis was placed on decreasing sedation levels and the use of deep sedation only in specific cases. The evidence and recommendations for the use of drugs such as dexmedetomidine, remifentanil, ketamine and others were incremented.
Dada la importancia del manejo de la sedación, analgesia y delirium en las unidades de cuidados intensivos, y con el fin de actualizar las guías publicadas anteriormente, se decidió elaborar una nueva guía de práctica clínica con los soportes, manejos e intervenciones más relevantes acordes con las publicaciones recientes. Para elaborar esta guía, se reunió un grupo de 24 intensivistas procedentes de 9 países de la Federación Panamericana e Ibérica de Sociedades de Medicina Crítica y Terapia Intensiva. Se acogió la propuesta del Grading of Recommendations Assessment, Development and Evaluation Working Group para emitir el grado de recomendación y evaluar la calidad de la evidencia. Se realizó una búsqueda sistemática de la literatura utilizándose: MEDLINE, las siguientes bases de datos de la biblioteca Cochrane: Cochrane Database of Systematic Reviews (CDSR), Cochrane Central Register of Controlled Trials (CENTRAL), Database of Abstracts of Reviews of Effects (DARE), National Health Service Economic Evaluation Database (NHS EED), y la base de datos de Literatura Latinoamericana y del Caribe en Ciencias de la Salud (LILACS). Finalmente, se seleccionaron 438 referencias, permitiendo realizar 47 recomendaciones fuertes con evidencia alta y moderada, 14 recomendaciones condicionales con evidencia moderada y 65 recomendaciones condicionales con evidencia baja. Se confirma la importancia del manejo inicial y multimodal del dolor, se hace énfasis en la disminución de los niveles de sedación y la utilización de sedación profunda solo en casos específicos. Aumenta la evidencia y recomendaciones para el uso de medicamentos como dexmedetomidina, remifentanil, ketamina, entre otros.
In 2007, the Pan-American and Iberian Federation of Societies of Critical Medicine and Intensive Therapy (Federación Panamericana e Ibérica de Sociedades de Medicina Crítica y Cuidados Intensivos [FEPIMCTI]) published the first evidence-based clinical practice guidelines for the management of sedoanalgesia in the critically ill adult patient.1 These guidelines were followed by a bilingual update (Spanish and English) in 2013.2 The success of these and other guides led to many similar publications by scientific societies such as the American Society of Critical Care Medicine, which have recently been updated.3
The studies supporting these documents and the awareness they have raised have resulted in changes in sedation and analgesia practices in the Intensive Care Unit (ICU). Some of these new practices are associated with significantly improved clinical outcomes.4 This explains why the updating of guides and the dissemination of recent advances are so important.
This new revision of the evidence-based clinical practice guidelines was made to update the recommendations referred to the management of sedation, analgesia and delirium in the critically ill adult patient, and once again was carried out in both languages: Spanish and English. The working group extensively covered the literature in the three aforementioned areas, and used the bilingual guidelines published in 2013 as a starting point.2 Due to the great extent of this guide, we herein present an executive summary of the document, with the full text as complementary material, accompanied by the justifications corresponding to each recommendation.
MethodologyIn 2017, the council of the FEPIMCTI invited its members to identify experts to represent them in the work group in charge of drafting the new guidelines. A total of 24 specialists in critical care medicine, with epidemiological and literature research support, conformed the final group. Their responsibilities included definition of the scope of the guidelines and of the topics to be dealt with, as well as development of the clinically relevant questions and issues in need of answers. Each question was assigned to two experts per topic. The main purpose of the created document included updating of the previously published evidence-based clinical practice guidelines for the management of sedoanalgesia in critically ill adult patients.2 The intended users of these guidelines are physicians, nurses, clinical pharmacologists and professionals in the numerous disciplines that form part of the multidisciplinary team in the ICUs implicated in the management of critically ill adult patents.
The relevant publications were identified by carrying out an electronic search of all the studies related with the proposed topics, taking as starting point the date on which the search corresponding to the previous guidelines ended.2 Use was made of MEDLINE through PUBMED (1 January 2012 to 31 April 2018) and the following Cochrane Library databases: Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials (CENTRAL), Database of Abstracts of Reviews of Effects, National Health Service Economic Evaluation Database through the Cochrane Library, as well as the Latin American and Caribbean Literature in Health Sciences (Literatura Latinoamericana y del Caribe en Ciencias de la Salud [LILACS]) database.
The guidelines adhered to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) Working Group5 for assessing the quality of the evidence and issuing the corresponding grade of recommendation. Likewise, use was made of the GRADE PRO tool for rating the evidence, and for summarizing and presenting the information in a concise manner, reducing subjectiveness of the recommendations, and facilitating decision making. Those recommendations with a voting rate of over 80% were included by consensus, while those that fell short of this percentage were withdrawn.
Lastly, the questions, recommendations and justifications were grouped into 6 different sections according to the specific conditions characterizing the group of patients to which they were addressed:
1. Benefits of sedoanalgesia
2. Sedation
- a
- a
Conscious sedation strategies
- b
ABCDEF bundle
- c
Recommendations for the use and duration of sedating agents
- d
Indications and strategies for deep sedation
- e
Impact of amnesia versus memory preservation
- f
Sedation in patients with ARDS and ECMO
- g
Sedation in patients with cardiovascular impairment
- h
Sedation in the neurocritical patient
- a
3. Analgesia
- a
- a
Benefits and strategies for the adequate management of pain in patients admitted to the UCI
- b
Analgesia in patients with cardiovascular impairment
- c
Analgesia in patients with sepsis and septic shock
- d
Analgesia in patients with ARDS and ECMO
- e
Analgesia in the oncological patient
- a
4. Delirium
- a
Identification, prevention and management of delirium
- b
Prediction of delirium
- c
Persistent cognitive deficit
5. Special populations
- a
Patients with renal or liver failure
- b
Patient analgesia in the postoperative period of cardiac, lung, liver and renal transplantation
6. Miscellaneous
- a
Trauma, pregnant, burn and elderly patients
- b
Withdrawal syndrome related to alcohol and other substances
The high level of evidence search including systematic reviews, meta-analyses, randomized trials and guides related to analgesia, sedation and delirium in critical patients generated a total of 4192 articles in the different databases cited above. Based on this search, the recommendations were classified according to level of evidence and grade of recommendation (GRADE) (Table 1). A total of 136 recommendations were finally made. The questions with their respective recommendations, strength and level of the evidence are summarized in Table 2.
Levels of evidence and grades of recommendation.
| Level of evidence | Description and implications |
|---|---|
| High level of evidence | It is sure that the real effect of the intervention comes close to the estimated effect |
| Moderate level of evidence | It is almost sure that the real effect of the intervention comes close to the estimated effect, but it is possible that the effect may be different |
| Low level of evidence | The certainty of the estimated effect is limited. The real effect is probably substantially different from the estimated effect |
| Very low level of evidence | There is little certainty with respect to the estimated effect. The real effect is substantially different from the estimated effect |
| Grade of recommendation | Description |
|---|---|
| Strong recommendation | There is certainty with respect to the desired effects. The intervention should be offered to all patients if favorable, or should not be used if not favorable |
| Conditional recommendation | There is no complete certainty with respect to the desired effect. Adherence to this recommendation probably has a greater impact upon the undesired effects, but there is not enough certainty in this respect |
Recommendations of the sedoanalgesia and delirium guidelines in critically ill adults.
| Question | Recommendation | Strength | Level of evidence |
|---|---|---|---|
| Benefits of sedoanalgesia | |||
| What are the short- and long-term benefits of adequate pain and sedation management in the critically ill adult patient? | A1. The suggestion is to adapt strategies designed first to prevent pain, followed by analgesic management in the early stages of pain and, if the addition of sedatives proves necessary, to administer them at the minimum effective dose | Conditional | Low |
| A2. The recommendation is to create or adapt protocols for adequate pain and sedation management, promoting the use of sedoanalgesia, mild sedation or no sedation in the context of care focused on the patient needs, and avoiding the use of benzodiazepines | Strong | Moderate | |
| A3. The recommendation is to use strategies allowing appropriate evaluation and adherence to pain and sedation management in all critical patients | Strong | Low | |
| What are the benefits of sedation, delirium and analgesia protocols? | A4. The suggestion is to use protocols for the evaluation and management of analgesia, agitation and delirium in order to improve the outcomes, such as adequate pain control, reduction of agitation and delirium episodes, lesser drug exposure, lesser time on mechanical ventilation, and shorter ICU and hospital stay | Conditional | Moderate |
| Sedation | |||
| What is the recommended sedation management for adult patients with sepsis and septic shock? | A1. The suggestion is to use mild sedation whenever possible in patients requiring mechanical ventilation, with periodic evaluation of their neurological condition, and the use of sedation scales according to individualized objectives. Routine deep sedation is to be avoided | Conditional | Low |
| What is the best sedation approach for critically ill adult patients without ventilatory support? | A2. In patients without orotracheal intubation and without ventilatory support, the suggestion is to use drugs with a low risk of causing respiratory depression or serious hemodynamic adverse effects, such as dexmedetomidine at low doses. The level of sedation should be monitored | Conditional | Low |
| What is the impact of applying the ABCDEF protocol in critically ill patients? | B1. The recommendation is to apply the ABCDEF protocol in critically ill patients to increase the number of days without delirium and the days without coma, and to reduce the duration of ventilatory support, intensive care stay and mortality | Strong | Low |
| What are the benefits of the presence of relatives in the outcome of the critically ill patient? | B2. The presence, participation and preparation of the family of the patient is suggested in the critical care plan as a psychosocial support measure | Conditional | Low |
| What are the benefits of early mobilization in critically ill patients? | B3. The recommendation is to implement passive mobilization of all patients in intensive care, followed by active mobilization when allowed by the clinical condition | Strong | Moderate |
| B4. Early mobilization is recommended in all surgical patients subjected to mechanical ventilation for at least 48hours. This must be based on an institutional protocol implicating physicians, nurses and therapists | Strong | Moderate | |
| B5. Early mobilization is recommended in all hemodynamically stable patients in the postoperative period of coronary revascularization or valve replacement surgery | Strong | Moderate | |
| Is there a relationship between benzodiazepine dose and time of use and the adverse effects of such drugs in critically ill patients? | C1. The suggestion is to avoid incrementing nocturnal midazolam doses, preferring the use of non-benzodiazepine drugs | Conditional | Low |
| C2. If sedation with midazolam is used, the suggestion is to provide mild rather than deep sedation in order to avoid delirium recall and not affect implicit patient memory | Conditional | Low | |
| C3. When midazolam is used for the management of sedation in the critical patient, the suggestion is to avoid the drug in continuous infusion and prefer intermittent bolus doses | Conditional | Low | |
| C4. The suggestion is to avoid deep sedation with midazolam | Conditional | Moderate | |
| C5. The recommendation is to avoid the use of benzodiazepines in patients at a high risk of suffering delirium | Strong | High | |
| When should benzodiazepines be used in the critically ill patient without alcohol abstinence? | C6. The use of midazolam rather than thiopental is suggested as part of the management of refractory convulsive states | Conditional | Low |
| C7. The addition of midazolam to haloperidol is suggested to improve agitation control in palliative patients | Conditional | Low | |
| What critically ill patients stand to benefit most from the use of remifentanil? | C8. The recommendation is to use remifentanil in the postoperative period of cardiac surgery to reduce the duration of mechanical ventilation | Strong | Moderate |
| C9. The suggestion is to use remifentanil in combination with propofol for sedation during therapeutic hypothermia after cardiac arrest | Conditional | Low | |
| C10. The suggestion is to use remifentanil in patients with renal failure to reduce the duration of mechanical ventilation and ICU stay | Conditional | Low | |
| C11. The suggestion is to use remifentanil in neurocritical patients to reduce waking time and allow neurological evaluation | Conditional | Low | |
| C12. The suggestion is to titrate remifentanil to the lowest effective dose possible in order to reduce hyperalgesia associated to use of the drug and its posterior suspension | Conditional | Low | |
| What is the association between the dose and duration of dexmedetomidine and its adverse effects? | C13. The recommendation is to avoid the generalized use of dexmedetomidine loading doses in critical patients | Strong | High |
| C14. When loading doses are required, the suggestion is to administer a dose of < 1μg/kg during > 20 minutes | Conditional | Low | |
| C15. The suggestion is to use sedatives other than dexmedetomidine when deeper sedation is required, rather than to administer loading doses of the latter drug | Conditional | Low | |
| C16. The recommendation is to avoid maintenance doses of > 1.4μg/kg/h and deep sedation levels based on dexmedetomidine in order to avoid the risk of severe bradycardia | Strong | Moderate | |
| C17. The suggestion is to use minimum doses of dexmedetomidine for mild sedation during maintenance, usually < 0.7μg/kg/h | Conditional | Moderate | |
| C18. The suggestion is to titrate sedatives other than dexmedetomidine when deep sedation is required, and to avoid doses of > 1.4μg/kg/h | Conditional | Low | |
| C19. In patients with bradycardia and hemodynamic alterations, the recommendation is to lower the maintenance dose or suspend it temporarily | Strong | Low | |
| C20. The suggestion is to periodically evaluate the need to continue dexmedetomidine infusion for a prolonged period of time (up to 7 days) | Conditional | Moderate | |
| C21. If needed, continue sedation with dexmedetomidine as maintenance for over 7 days, since there is not enough evidence to recommend a maximum time. It is advisable to alternate with periods of drug suspension and to monitor the appearance of adverse effects | Conditional | Low | |
| C22. The suggestion is to temporarily suspend dexmedetomidine infusion when hyperthermia associated to its use is suspected, particularly in obese patients and in the postoperative period of cardiovascular surgery | Conditional | Low | |
| What is the recommended drug strategy to maintain a Richmond Agitation-Sedation Scale (RASS) score of -4 or -5 in patients requiring sedation for over 72 hours? | D1. The recommendation is to combine a hypnotic with an analgesic (preferably an opiate) to secure deep sedation, provided it is clinically justified | Strong | Moderate |
| D2. The suggestion is to use midazolam in prolonged deep sedation and/or to combine it with an opiate, propofol and/or dexmedetomidine | Conditional | Moderate | |
| D3. The suggestion is to use inhalation sedation as an alternative to deep intravenous sedation in cases of status asthmaticus, status epilepticus or respiratory difficulty | Conditional | Low | |
| What is the current impact of amnesia versus memory preservation in critically ill adult patients? | E1. The recommendation is to promote the recall of positive events and avoid complete amnesia in order to reduce post-intensive care syndrome (PICS) and improve patient functional outcome after ICU discharge | Strong | Low |
| E2. The recommendation is to administer mild sedation in the critical patient in order to reduce recall associated to sensory-perceptive disorders (delusions or hallucinations) | Strong | Moderate | |
| E3. The suggestion is to keep a log of critical patient ICU stay, within an integral post-ICU neurocognitive rehabilitation plan | Conditional | Low | |
| What is the recommended sedation management for adult patients with acute respiratory distress syndrome (ARDS)? | F1. In patients with ARDS and PaO2/FiO2 ≥ 150, the recommendation is to follow the guidelines, with conscious or cooperative sedation whenever possible, adopting the recommendations for patients on mechanical ventilation, with periodic evaluation of the sedation level using scales, and following the nursing guided sedation protocols. In patients with moderate oxygenation disorders characterized by PaO2/FiO2<150 and who require muscle relaxation, deep sedation is suggested | Strong | Moderate |
| What are the best sedation strategies in patients subjected to extracorporeal membrane oxygenation (ECMO)? | F2 The suggestion is to adjust the dosage of sedatives (propofol, midazolam or dexmedetomidine), since their pharmacokinetics are altered by the circuit components. In turn, ketamine is suggested in order to reduce the sedoanalgesia and vasopressor doses | Conditional | Low |
| What is the recommended sedation management for adult patients with hemodynamic instability? | G1. The suggestion is to use ketamine as coadjuvant to the sedation strategy in hemodynamically unstable patients | Conditional | Low |
| G2. Cautious use of sedatives is recommended in hemodynamically unstable patients | Strong | Moderate | |
| What is the best sedation management in patients with coronary disease? | G3. Dexmedetomidine for sedation is suggested in patients with acute coronary syndrome | Conditional | Low |
| G4. In the postoperative period of myocardial revascularization, dexmedetomidine is suggested as the drug of choice for sedation | Strong | High | |
| G5. Caution is suggested with the use of dexmedetomidine and alpha-2-agonists, since they pose a risk of arterial hypotension and bradycardia | Conditional | Moderate | |
| What are the recommended analgesia and sedation strategies during electrical cardioversion? | G6. The recommendation is to use propofol and midazolam as drugs of choice for electrical cardioversion | Strong | Moderate |
| G7. The suggested propofol dose ranges from 0.5-1mg/kg administered in 30-60 seconds; the addition of a low-dose opiate (alfentanil 5μg/kg, remifentanil 0.25μg/kg) is a safe alternative, and is not associated to greater complications | Conditional | Moderate | |
| G8. Etomidate appears to be similar to propofol during electrical cardioversion, but is associated to a greater incidence of adverse events. It is therefore regarded as a second line option. Midazolam is effective during electrical cardioversion, but requires monitoring for a longer period after cardioversion, and flumazenil must be available as antidote | Conditional | Low | |
| G9. The chosen drug may be administered by a physician with adequate equipment and training in management of the airway. In this scenario, midazolam is the drug of choice | Conditional | Moderate | |
| G10. The suggestion is to administer dexmedetomidine at a dose of 1μg/kg in 10minutes before administering a sedative, in order to reduce arrhythmia relapse in the first 24hours | Conditional | Low | |
| What is the recommended sedation management for adult patients with intracranial hypertension? | H1. The recommendation is to apply a sedation strategy in all patients with intracranial hypertension, in order to afford brain protection | Strong | Moderate |
| H2. The suggestion is to use barbiturates such as thiopental sodium or pentobarbital only in cases of intracranial hypertension refractory to other therapeutic measures | Conditional | Low | |
| H3. The daily interruption of sedation in patients with intracranial hypertension is not recommended | Conditional | Low | |
| What is the recommended sedation management for adult patients with severe traumatic brain injury? | H4. The suggestion is to use drugs with a short half-life and scant accumulation (propofol, dexmedetomidine and remifentanil), allowing frequent neurological evaluations | Conditional | Low |
| In which patients requiring sedation is the use of brain activity monitoring devices indicated? | H5. The recommendation is to use frontal brain activity electronic monitoring systems in patients under the effects of neuromuscular relaxation, in order to avoid under- and oversedation | Strong | Moderate |
| H6. The suggestion is to use frontal brain activity electronic monitoring systems in order to reduce the sedative dose in patients subjected to deep sedation | Conditional | Low | |
| H7. The use of validated clinical scales is suggested to assess sedation/agitation level in critical patients under mild sedation and without neuromuscular block | Conditional | Very low | |
| Analgesia | |||
| What are the benefits of using analgesia protocols? | A1. The recommendation is to use analgesia and sedation protocols based on analgesics for adequate pain control in all critical patients admitted to the ICU | Strong | Moderate |
| A2. Continuous education and capacitation of the staff in charge of patient care (nurses, intensivists, therapists) is recommended regarding the protocol and treatment options available in the center | Strong | Moderate | |
| What are the analgesia strategies in the critical patient? | A3. The recommendation is to always assess pain using scales in accordance to the patient conditions | Strong | Moderate |
| A4. The recommendation is to provide clear instructions on the evaluation, intervention, objectives and side effects of the therapy to be applied | Strong | Low | |
| A5. Opioid analgesics are suggested as part of one of the first lines of analgesic treatment for pain of non-neuropathic origin | Conditional | Moderate | |
| A6. If opioids are administered, the suggestion is to use the lowest dose possible to keep the patient comfortable | Conditional | Low | |
| A7. Periodic pain evaluation is recommended in order to allow adequate dose adjustment | Strong | Moderate | |
| A8. Analgesic administration is recommended before carrying out procedures that exacerbate pain | Strong | Moderate | |
| A9. The use of non-pharmacological measures such as music therapy, mindfulness, electrostimulation and massages, is suggested as coadjuvant therapy | Strong | Moderate | |
| A10. The adoption of a multimodal strategy and/or the ABCDEF bundle is suggested for promoting early activation of the critical patient in the ICU | Strong | Moderate | |
| What are the benefits of optimizing opioid use in the critically ill, and what patients stand to benefit most? | A11. The recommendation is to use the lowest opioid doses possible to secure the therapeutic objective, and only for the shortest time possible | Strong | Moderate |
| What is the recommended pain management strategy for the critical patient without ventilatory support? | A12. Routine pain monitoring in the ICU is recommended, using a validated tool, in order to improve pain management and ensure more efficient analgesic use | Strong | Moderate |
| A13. The recommendation is to stratify patients according to the type of pain (e.g., acute, subacute, chronic, neuropathic or non-neuropathic) and its intensity (e.g., mild 0-3/10 or moderate to severe > 4/10), as well as according to prior exposure to opioids (e.g., first exposure versus tolerant patient), in order to choose the best therapeutic option | Strong | Moderate | |
| A14. The recommendation is to use multimodal analgesia with the aim of controlling pain in the critical patient not subjected to mechanical ventilation, and to reduce opioid use in such individuals | Strong | Moderate | |
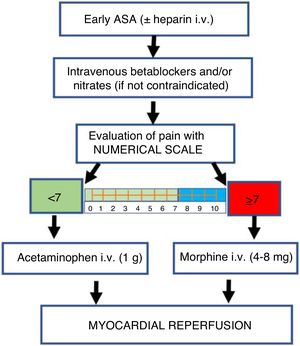
| What is the recommended pain management for patients with coronary disease? | B1. The recommendation is to avoid the routine use of morphine in patients with acute myocardial infarction | Strong | Moderate |
| B2. The recommendation is to adopt pain control strategies other than morphine in patients with myocardial infarction, including the use of nitrates and beta-blockers | Strong | Moderate | |
| B3. The suggestion is to restrict morphine use in patients with ST-segment elevation myocardial infarction in cases of persistent severe pain (visual analog scale [VAS] ≥ 7 points), despite the start of anti-ischemia and antithrombotic therapies | Conditional | Low | |
| B4. Acetaminophen is suggested as analgesic strategy in hypertensive patients with cardiovascular risk | Conditional | Low | |
| B5. The avoidance of nonsteroidal antiinflammatory drug (NSAID) use is suggested as prolonged analgesia strategy in chronic hypertensive patients with coronary disease | Conditional | Low | |
| B6. Ketamine is suggested as part of the analgesic strategies in hemodynamically unstable patients | Conditional | Moderate | |
| B7. Cautious use of intravenous acetaminophen is advised in hemodynamically unstable patients | Conditional | Low | |
| What is the recommended analgesic management for adult patients with sepsis and septic shock? | C1. Routine pain monitoring in the ICU is recommended, using a validated tool, in order to improve pain management and ensure more efficient analgesic use | Strong | Moderate |
| C2. The suggestion is to stratify patients according to the type of pain (e.g., acute, subacute, chronic, neuropathic or non-neuropathic) and its intensity (e.g., mild 0-3/10 or moderate to severe > 4/10), as well as according to prior exposure to opioids (e.g., first exposure versus tolerant patient), in order to choose the best therapeutic option | Conditional | Moderate | |
| C3. The suggestion is to use multimodal analgesia with the aim of controlling pain in the critical patient not subjected to mechanical ventilation, and to reduce opioid use in such individuals | Conditional | Moderate | |
| What is the recommended analgesic management strategy for adult patients with ARDS? | D1. The recommendation is to use opioids (with particular consideration of remifentanil) to afford acute pain relief. The dosage should be adjusted according to the stage of the disease process and the specific needs of patients with severe ARDS | Strong | Moderate |
| D2. The avoidance of high-dose remifentanil is suggested in order to reduce controversial hyperalgesia associated to use of the drug | Conditional | Low | |
| What is the recommended analgesic management strategy for patients subjected to ECMO? | D3. The suggestion is to avoid the use of lipophilic analgesics (e.g., fentanyl) due to the degree of circuit trapping involved | Conditional | Low |
| D4. The use of preferably non-lipophilic analgesics is suggested (e.g., morphine) | Conditional | Low | |
| D5. The suggestion is to adjust the dosage of analgesics according to the time on ECMO, circuit status (new or old), and whether venovenous or venoarterial ECMO is used | Conditional | Low | |
| Should the presence of pain be evaluated in end-of-life patients subjected to limitation of therapeutic effort (LTE)? | E1. The suggestion is to evaluate the presence of pain, agitation and breathing difficulty on a protocolized basis in end-of-life patients. In the case of patients with communication difficulties, the evaluation of objective signs of pain and breathing difficulty is suggested | Conditional | Low |
| What is the aim of analgesic treatment in end-of-life patients subjected to limitation of therapeutic effort (LTE)? | E2. The suggested aim of drug treatment during LTE is to prevent and treat pain and other symptoms such as distress or breathing difficulty When a treatment is administered, it is advised to register justification of its use | Conditional | Low |
| Delirium | |||
| What are the short- and long-term benefits of adequate delirium management in the critically ill adult patient? | A1. Adequate management of delirium is recommended, since over the short term it reduces the duration of mechanical ventilation, cognitive disorders, and ICU and hospital stay. Furthermore, over the long term it is associated to lesser mortality and improved quality of life | Strong | High |
| A2. Dexmedetomidine in continuous infusion is recommended in patients with hyperactive delirium subjected to mechanical ventilation, since it is associated to more ventilator-free hours in the first 7 days, earlier extubation and faster resolution of delirium. | Strong | Moderate | |
| What are the benefits of using delirium detection and management strategies? | A3. Daily assessment using a validated scale such as the CAM-ICU (the confusion assessment method for the intensive care unit) and ICDSC (intensive care delirium screening checklist) is recommended for the detection of delirium in critically ill patients with or without mechanical ventilation, since it is associated to reduced mortality and hospital stay | Strong | Moderate |
| A4. Multicomponent interventions are recommended (lowering of light and noise, covering of the eyes, frequent patient orientation and music) to reduce the duration in days and improve the outcomes | Strong | Moderate | |
| What are the most recommended pharmacological and non-pharmacological measures for preventing delirium in the critical patient? | A5. Structural, organizational and medical management efforts are recommended to reduce anxiety, improve patient adaptation and comfort and contribute to adequate pain control, together with frequent reorientation and optimization of the environment in order to reduce the appearance of delirium | Strong | Moderate |
| A6. Multimodal interventions are recommended (lowering of light and noise, covering of the eyes, frequent patient orientation and music), together with the ABCDEF strategy in critically ill patients | Strong | Moderate | |
| A7. Integration of the family with unrestricted visits for the prevention of delirium is suggested within the context of non-pharmacological therapy | Conditional | Low | |
| A8. The recommendation is to use low-dose dexmedetomidine in continuous infusion in patients in the postoperative period of non-cardiac surgery with a high risk of suffering delirium | Strong | High | |
| A9. The administration of dexmedetomidine is recommended in patients subjected to noninvasive ventilation, in order to prevent delirium and reduce the need for intubation | Strong | Moderate | |
| A10. The use of haloperidol is suggested for the prevention of delirium in patients over 75 years of age in the postoperative period of abdominal and orthopedic surgery | Conditional | Moderate | |
| What are the most recommended pharmacological and non-pharmacological measures for the treatment of delirium in the critical patient? | A11. The ABCDEF and multimodal strategies are recommended for delirium management in the critically ill patient | Strong | Moderate |
| A12. The recommendation is to use dexmedetomidine for the management of patients with hyperactive delirium subjected to mechanical ventilation | Strong | Moderate | |
| A13. The use of quetiapine is suggested in patients with hyperactive delirium | Conditional | Low | |
| A14. The use of dexmedetomidine is recommended in critical patients with delirium | Strong | Moderate | |
| Is it possible to predict the appearance of delirium in the critical patient? | B1. The recommendation is to use the E-PRE-DELIRIC and PRE-DELIRIC models upon admission and after 24hours of admission, respectively, to predict the risk of delirium | Strong | Moderate |
| B2. The recommendation is to use the NICE predictive rules, the APREDEL-ICU, the AWOL tool and other models based on risk factors, to predict the risk of delirium | Strong | Low | |
| What are the risk factors associated to persistent cognitive deficit? | C1. The recommendation is to assess the risk factors associated to the appearance of persistent cognitive deficit in patients admitted to the ICU. | Strong | Low |
| The main risk factors associated to persistent cognitive deficit are the presence of delirium in the ICU and posttraumatic stress symptoms during the first 30 days after discharge | |||
| What are the strategies for preventing persistent cognitive deficit? | C2. The prevention and management of delirium is recommended as the main strategy for reducing the incidence of persistent cognitive deficit | Strong | Low |
| C3. The suggestion is to promote early mobilization, reduce sedatives, optimize sleep and provide adequate emotional and psychological support as strategies associated to the decrease in persistent cognitive deficit | Conditional | Low | |
| Special populations | |||
| What analgesics and sedatives are indicated and contraindicated in patients with liver failure? | D1. The suggestion is to use multimodal analgesia for the treatment of postoperative pain in adult patients with renal or liver failure | Conditional | Low |
| D2. The use of NSAIDs in patients with liver failure is not advised | Conditional | Low | |
| D3. The suggestion is to use acetaminophen as first step treatment for acute pain in patients with non-alcoholic cirrhosis, at a dose of 2-3g/day | Conditional | Low | |
| D4. The use of dipyrone in cirrhotic patients is not advised | Conditional | Low | |
| D5. The use of tramadol at a dose of 25mg every 8hours is suggested as second line treatment after acetaminophen | Conditional | Low | |
| What analgesics and sedatives are indicated and contraindicated in patients with renal failure? | D6. The use of fentanyl and methadone is suggested as safe options in patients with renal failure | Conditional | Low |
| D7. The suggestion is to use hydromorphone and oxycodone with caution, reducing the dose in patients with renal failure | Conditional | Low | |
| D8. The use of codeine, hydrocodone, meperidine and morphine is not advised in patients with renal failure | Conditional | Low | |
| D9. The suggestion is to use acetaminophen, increasing the dosing interval to once every 8hours, and administering doses of no more than one gram when the glomerular filtration rate (GFR) is <10ml/minute | Conditional | Low | |
| D10. The administration of tramadol is suggested for the management of pain, at doses of 100mg every 12hours in patients with an estimated GFR of 30ml/minute, and 50mg every 12hours when the estimated GFR is < 10ml/minute | Conditional | Low | |
| D11. The use of NSAIDs is not advised in patients with renal failure | Conditional | Low | |
| What are the recommended drugs for postoperative analgesia in heart, lung, liver or kidney transplant patients? | E1. The suggestion is to provide analgesia in the postoperative period of lung transplantation using paravertebral block or continuous thoracic epidural analgesia | Conditional | Low |
| E2. Caution is advised when using dexmedetomidine as sedative in patients during the postoperative period of lung transplantation, due to the risk of asystole | Conditional | Low | |
| E3. Intraoperative control and administration of magnesium is suggested when tramadol is used as postoperative analgesic | Conditional | Low | |
| E4. Ultrasound-guided subcostal transversus abdominis plane block (STAP) is suggested in the management of pain during the postoperative period of liver transplantation | Conditional | Low | |
| E5. The use of NSAIDs is not advised in the postoperative period of renal transplantation | Conditional | Low | |
| E6. The suggestion is to use acetaminophen as first step treatment for the management of renal post-transplantation pain | Conditional | Low | |
| E7. Transverse abdominis plane (TAP) block is not advised for the management of renal post-transplantation pain, since it does not affect the use of post-transplantation morphine | Conditional | Low | |
| E8. The use tramadol is suggested in the postoperative period of renal transplantation | Conditional | Low | |
| E9. The use of hydromorphone is advised in the immediate postoperative period of renal transplantation | Conditional | Low | |
| Miscellaneous | |||
| What are the special considerations and pharmacological recommendations for the management of sedation and analgesia in special situations (trauma, elderly patients, burn victims and pregnant women)? | A1. The use of dexmedetomidine is suggested as an alternative to haloperidol in the management of delirium in trauma cases without traumatic brain injury (TBI) | Conditional | Moderate |
| A2. In patients without acute brain damage, the recommendation is to use ketamine as additional analgesic in cases of chest trauma and rib fractures where pain control is not achieved with patient-controlled analgesia (PCA) or regional techniques | Conditional | Moderate | |
| A3. Monitoring with the bispectral index (BIS) is recommended in the management of multiple trauma patients | Strong | Moderate | |
| A4. The suggestion is to start methadone in the first four days of mechanical ventilation in order to reduce the ventilation times in patients that possibly may be ventilated for at least one week | Conditional | Low | |
| A5. The use of dexmedetomidine is recommended for the prevention of delirium in elderly patients following non-cardiac surgery | Strong | High | |
| A6. The use of dexmedetomidine is recommended as sedation in the perioperative period of cardiac surgery in patients over 60 years of age to prevent delirium | Strong | Moderate | |
| A7. Dexmedetomidine is recommended as sedative of choice in patients with postpartum eclampsia requiring mechanical ventilation, in relation to a decrease in arterial pressure, heart rate, a lessened need for antihypertensive drugs and shorter ICU stay | Strong | Low | |
| A8. The use of dexmedetomidine as coadjuvant is recommended for the sedation of ventilated burn victims | Conditional | Low | |
In developing this new version of the guidelines, questions were raised involving issues that had not been addressed in the previous guidelines. Other questions were referred to topics that had already been considered, but which were believed to merit an update. The working group examined the best available evidence to answer these questions, and found that only 6 of them had a high level of evidence—the level being moderate and low for the great majority of the questions. The strength of the recommendations depended not only on the quality of the available evidence (this being the most important criterion) but also on the relevance attributed by the expert consensus to each intervention. In this way, some strong recommendations were made, with many more conditional recommendations.
The strong recommendations were based on 7 points:
- 1
Evaluation of pain: Emphasis is placed on the importance of adequate pain evaluation using scales for each scenario and type of patient, and on offering optimum management and follow-up.
- 2
Education: This refers to information for the patient and family about the intervention to be made, its indications, consequences, advantages, limitations and risks.
- 3
Opioids and multimodal analgesia: In patients with moderate to severe pain, opioids remain the first line treatment. However, the adverse effects of opioid use and abuse are increasingly recognized; different analgesic alternatives therefore need to be found in the context of a multimodal strategy, with the purpose of reducing exposure to these drugs.
- 4
Mild sedation: Due evaluation is required of whether each individual patient requires sedation or not, with the purpose of affording comfort. In this regard, sedation should be kept as superficial as possible, prescribing deep sedation only where indicated, and when the existing evidence has demonstrated benefits.
- 5
Delirium: This problem should be addressed in the critical patient from the time of admission, with the prediction of risk, prevention, detection and management. In relation to prevention and management, different pharmacological and non-pharmacological measures are available that have demonstrated benefits with levels of evidence ranging from high to low.
- 6
Early mobilization: Deconditioning in the ICU should be reduced through early mobilization (passive and active) when allowed by the patient condition, based on protocols, shortening of stays and improving functional independence and patient quality of life upon discharge.
- 7
Reduced sleep disruption: Quality sleep with less fragmentation is indicated. Non-pharmacological measures such as reducing noise and illumination at night, among other options, have better evidence than pharmacological measures.
Other recommendations were presented in this document as conditional. However, this does not mean that such interventions are not important, for although the supporting evidence was weaker, the working group considered it opportune to include them in the benefit of the critical patient. Clinical judgment at the patient bedside, with due knowledge of the best strategies, contributes to make better decisions (Fig. 1).
ExonerationIt is important to underscore that guidelines are only a useful tool for improving medical decisions, and must be used with due consideration of medical criterion, the clinical circumstances, the patient preferences and the locally available resources. It also should be remembered that the novel findings of clinical research can contribute new evidence, making it necessary to modify standard practices even before the guidelines are updated.
FundingDevelopment of the guidelines has been possible thanks to the unconditional support of the Pan-American and Iberian Federation of Societies of Critical Medicine and Intensive Therapy (Federación Panamericana e Ibérica de Sociedades de Medicina Crítica y Cuidados Intensivos [FEPIMCTI]), the Hospital Universitario Fundación Santa Fe de Bogotá (FSFB), the Colombian Association of Critical Medicine and Intensive Care (Asociación Colombiana de Medicina Crítica y Cuidado Intensivo [AMCI]), and an unrestricted educational grant from Pfizer.
Conflicts of interestThe members of the consensus group declare the following conflicts of interest: J.C. Diaz, principal investigator or co-investigator in studies sponsored by Abbot, Aspen, Amarey, Glaxo, speaker for Aspen, Baxter, B.Braun, Dräger, Hamilton, Hospira and Glaxo; G. Castorena, speaker for MSD in relation to sugammadex; G. Castillo Abrego, speaker for Medtronic and Zoll Medical; J.M. Pardo, support from MSD and Sanofi Aventis for participation in congresses. The rest of the authors declare that they have no conflicts of interest.
Thanks are due to the Colombian Association of Critical Medicine and Intensive Care (Asociación Colombiana de Medicina Crítica y Cuidado Intensivo [AMCI]) for its support of the preparation of the guidelines.
Thanks are also due to Hospital Universitario Fundación Santa Fe de Bogotá (FSFB) for its academic and administrative support in the preparation of the guidelines, and especially to Alexandra Suárez for her help in organization of the logistics and secretarial work involved in drafting the guidelines.
Please cite this article as: Celis-Rodríguez E, Díaz Cortés JC, Cárdenas Bolívar YR, Carrizosa González JA, Pinilla D.I., Ferrer Záccaro LE, et al. Guías de práctica clínica basadas en la evidencia para el manejo de la sedoanalgesia y delirium en el paciente adulto críticamente enfermo. Med Intensiva. 2020;44:171–184.