To study the results of a non-controlled cardiac death (Maastricht type II) donor program in a city of 200,000 inhabitants. The study was initially focused on lung donation and was extended to kidney donation after 9 months.
DesignA prospective observational study was conducted between October 2012 and December 2013.
SettingThe Intensive Care Unit of Marqués de Valdecilla University Hospital in Santander (Spain), and surrounding areas.
PopulationsPatients (<55 years) who died of out-of-hospital cardiac arrest.
InterventionsAll out-of-hospital cardiac arrests were treated with mechanical cardiac compression (LUCAS II). The diagnosis of death and organ preservation were performed in the ICU.
ResultsA total of 14 calls were received, of which three were discarded. Of the 11 potential donors, 7 were effective donors with a median age of 39.5 years (range: 32–48). A total of 5 single lung transplants and four kidney transplants were performed. In addition, corneas and tissues were harvested. The non-valid donors were rejected mainly due to technical problems. There were no donation refusals on the part of the patient relatives. The lung transplant patient survival rate was 100% after one month and 80% after one year. One month after transplantation, the kidney recipients had a serum creatinine concentration of <2mg/dl. The interval from cardiac arrest to renal preservation was 80min (range: 71–89), and the interval from cardiac arrest to lung preservation was 84min (range: 77–94).
ConclusionsA Maastricht type II donation program in a small city is viable for both abdominal and thoracic organs. The program was initially very cautious, but its potential is easily improvable by increasing donor and by equipping mobile ICU ambulances with mechanical cardiac compression systems. Full management of the donor in the ICU, avoiding the emergency department or operating rooms, reduces the warm ischemia time, thereby improving transplant outcomes.
Analizar los resultados de la implantación de un programa de donación Maastricht II en una ciudad de 200.000 habitantes. Inicialmente solo donación pulmonar y tras 9 meses se amplió a donación renal.
DiseñoEstudio observacional prospectivo de octubre de 2012 a diciembre de 2013.
ÁmbitoUCI del Hospital Universitario Marqués de Valdecilla y área metropolitana de Santander.
PoblaciónPacientes<55 años fallecidos por parada cardiaca extrahospitalaria.
IntervenciónLa asistencia extrahospitalaria fue con cardiocompresor mecánico (LUCAS II). El diagnóstico de muerte, la asistencia y preservación de los injertos a donar se realizó íntegramente en la UCI.
ResultadosSe recibieron un total de 14 llamadas, descartándose 3. De los 11 potenciales donantes, 7 fueron donantes utilizados con edad mediana de 39,5 años (rango: 32–48). Se realizaron 5 trasplantes unipulmonares, 4 trasplantes renales, además de córneas y tejidos. Los donantes no válidos se debieron a problemas técnicos. No hubo negativas. La supervivencia de los trasplantados pulmonares fue 100% al mes y 80% al año. Todos los trasplantados renales presentaban creatinina al mes<2mg/dl. El tiempo parada-preservación renal fue 80 minutos (rango intercuartílico: 71–89) y el tiempo parada-preservación pulmonar fue 84 minutos (rango intercuartílico: 77–94).
ConclusionesUn programa Maastricht II en una ciudad pequeña es viable tanto para órganos abdominales como torácicos. La potencialidad es mejorable al incrementar la edad de valoración y disponer de cardiocompresores mecánicos en todas las ambulancias. El tratamiento íntegro del donante en la UCI reduce los tiempos de isquemia caliente mejorando los resultados postrasplante.
Donation under conditions of brain death has been the most widely adopted strategy for obtaining organs. In Spain, 90% of all donors correspond to individuals in which death has been certified according to neurological criteria.1 However, this type of donation has decreased as a result of epidemiological factors, including fewer traffic accidents, fewer occupational accidents, improved management of cerebrovascular risk factors, and more widespread application of novel neurosurgical techniques such as decompressive craniectomy. For this reason, new strategies are needed with a view to expanding the pool of organ donors, such as non-heart beating donation.
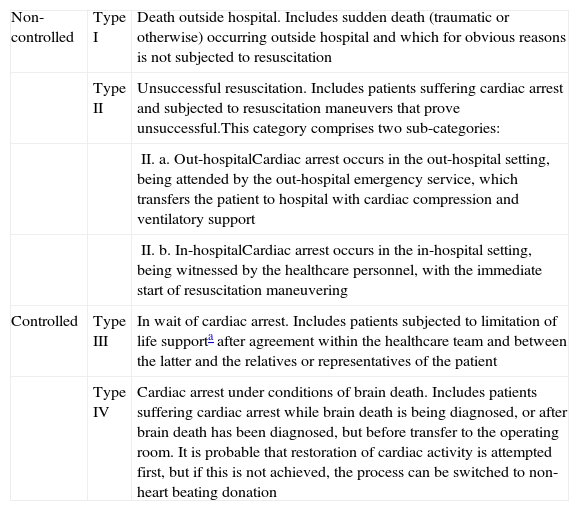
Maastricht type II non-heart beating donation (Table 1) has complex logistic requirements, with the simultaneous participation of many professionals. The latter in turn must be highly qualified, due to the narrow time margins available for making decisions.2 Traditionally, this type of donation has been limited to cities with large populations (over 500,000 inhabitants), though very recently non-heart beating donation programs such as those developed in Granada and Alicante (Spain) have shown that this type of donation is also viable in small- to middle-sized cities.3
Types of non-heart beating donation according to the Maastricht criteria (modification of Madrid 2011).
| Non-controlled | Type I | Death outside hospital. Includes sudden death (traumatic or otherwise) occurring outside hospital and which for obvious reasons is not subjected to resuscitation |
| Type II | Unsuccessful resuscitation. Includes patients suffering cardiac arrest and subjected to resuscitation maneuvers that prove unsuccessful.This category comprises two sub-categories: | |
| II. a. Out-hospitalCardiac arrest occurs in the out-hospital setting, being attended by the out-hospital emergency service, which transfers the patient to hospital with cardiac compression and ventilatory support | ||
| II. b. In-hospitalCardiac arrest occurs in the in-hospital setting, being witnessed by the healthcare personnel, with the immediate start of resuscitation maneuvering | ||
| Controlled | Type III | In wait of cardiac arrest. Includes patients subjected to limitation of life supporta after agreement within the healthcare team and between the latter and the relatives or representatives of the patient |
| Type IV | Cardiac arrest under conditions of brain death. Includes patients suffering cardiac arrest while brain death is being diagnosed, or after brain death has been diagnosed, but before transfer to the operating room. It is probable that restoration of cardiac activity is attempted first, but if this is not achieved, the process can be switched to non-heart beating donation |
The organs obtained from Maastricht type II programs are fundamentally kidneys and, to a lesser extent, liver grafts. To date, only the programs in San Carlos Hospital and Doce de Octubre Hospital have been able to harvest and transplant lung grafts.4
Recently, our group has designed and implemented a Maastricht type II non-heart beating donation program in the city of Santander (Spain) and its surroundings (225,000 inhabitants). A distinguishing feature of this program is that the diagnostic and organ preservation procedures are entirely performed in the Intensive Care Unit (ICU), thus making it possible to shorten the warm ischemia times, facilitate and simplify decision making, and save costs–since the program makes use of staff members on duty in the hospital itself and involved in care activities other than transplant coordination. This program is unique in Spain, and the fact that it is integrally developed in the ICU has allowed the successful obtainment of lung and kidney grafts. The present study describes the preliminary results obtained in the first year of the program.
Patients and methodsA prospective study was made of the activity of the non-controlled cardiac death (Maastricht type II) donation program in the Department of Intensive Care Medicine of Marqués de Valdecilla University Hospital in Santander, during the period between October 2012 and December 2013.
The activity of the program was documented, including the number of activations (potential donors), the number of effective and used donors, demographic information on the donors, out- and in-hospital times, causes of graft loss, and subsequent evolution of the transplant patients. The variables are reported as absolute values or percentages, or as the mean or median and interquartile range (IQR P25–P75). The data referred to the national series are reported as the mean and standard deviation.
Characteristics of the programThe exclusively pulmonary Maastricht type II non-heart beating donation program in the city of Santander was started in October 2012, and was expanded after the first 9 months to include kidney donation.
The program only contemplated out-hospital cardiac arrest, its area of influence corresponding to the city of Santander (177,123 inhabitants in January 2013) and a metropolitan area with 50,000 inhabitants.5
The program only accepted donors maintained by mechanical cardiac compression (LUCAS II® model); only two of the four mobile ICU ambulances in our Autonomous Community are equipped with this type of system.
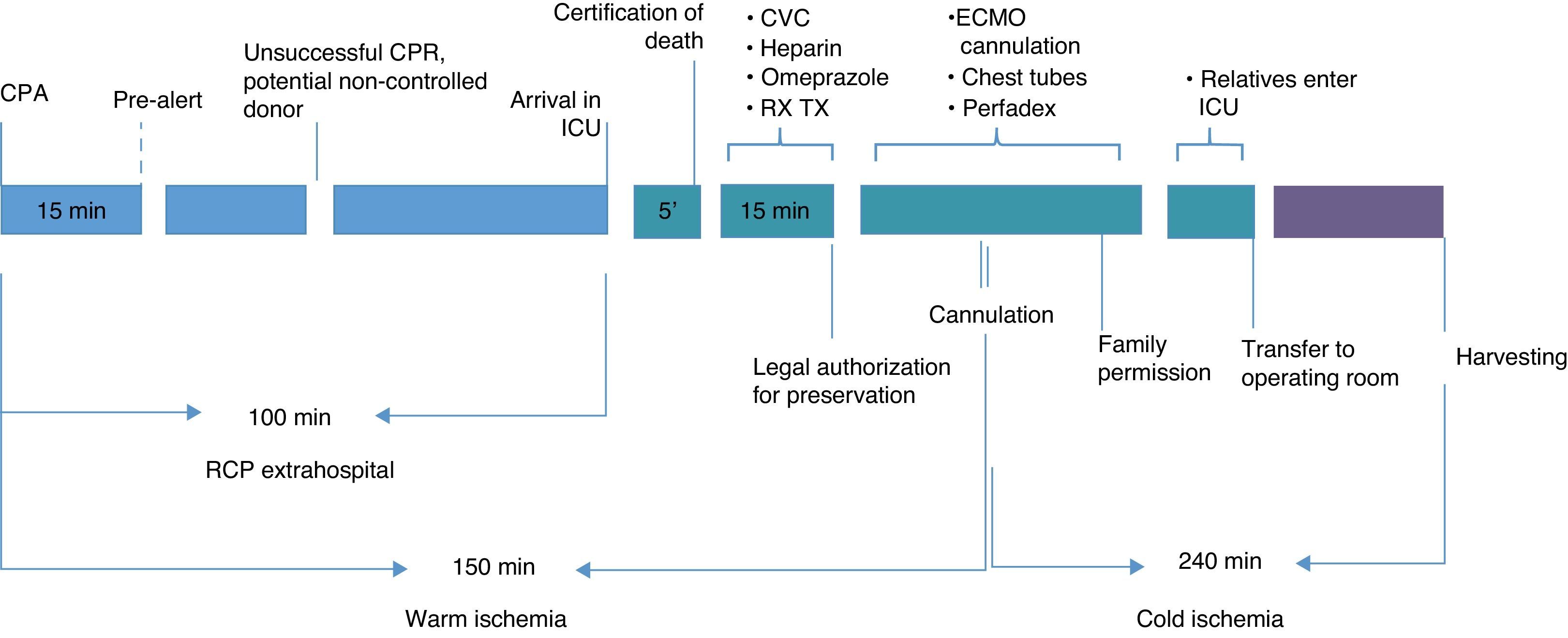
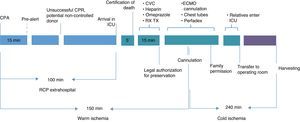
The chain of care referred to patients of this kind comprises two clearly distinct phases: out-hospital and in-hospital (Fig. 1).
The out-hospital phase is characterized by the following activities:
- (1)
Determination of the time of cardiorespiratory arrest from the witnesses.
- (2)
After at least 30min of advanced live support (ALS) without the recovery of an effective heart beat, the 061 team contacts the transplant coordinator and a joint evaluation is made based on the initial on site inclusion criteria (Table 2). After deciding donor viability, the non-heart beating donor protocol (“code 9”) is activated. Such activation can only be made by the transplant coordinator. At this point the donation of kidney or lung, or both, is considered.
Table 2.Activation criteria following on site evaluation of the potential non-controlled non-heart beating donor.
Potential donor activation criteria following unsuccessful ACPR Age 9–55 years Absence of chest injuries involving massive bleeding, total exsanguination Normal external appearance (exclusion of homeless individuals, suspected risk practices, vein punctures, death occurring in brothels, etc.) Time from cardiac arrest to start of advanced life support under 15min Time from cardiac arrest to arrival in ICU of Marqués de Valdecilla Hospital under 100min ACPR, advanced cardiopulmonary resuscitation.
- (3)
The potential donor is taken to hospital under mechanical ventilation and with external mechanical cardiac compression (LUCAS II®).
- (4)
Bleeding control is performed during transfer to hospital. From this time no drugs or fluids are administered, though access catheterization is maintained.
- (5)
Activation of the program by the transplant coordinator implies immediate transfer of the potential donor to the ICU of Marqués de Valdecilla University Hospital and activation of the fast response team (transplant nursing coordinator, ICU intensivist on duty, and two transplant team nurses).
If kidney donation is contemplated, the cardiovascular surgeon on duty and the perfusion nurse are alerted.
The in-hospital phase is entirely conducted in the ICU, and is characterized by the following activities:
- (1)
On arriving in the ICU without passing through emergency care, the intensivist and his or her team take charge of the patient, followed by disconnection of the respirator and cardiac compression system.
- (2)
After 5min without a pulse and/or in the absence of an electrocardiographic tracing, the intensivist on duty certifies death in asystole. A chest X-ray is obtained in those 5min. From that moment onwards, the transplant coordinator leads the process and is in charge of all the decisions, tests and treatments referred to the donor.
- (3)
Cardiac massage and ventilation are resumed until legal permission is obtained for organ preservation.
- (4)
In this period a central vascular access is prepared (jugular vein if kidney donation is contemplated; femoral vein if only lung harvesting is considered), blood samples are collected, and the following intravenous medication is administered: heparin sodium (1000U/kg in the case of kidney donation; 800U/kg if only lung donation is considered), 250ml of bicarbonate 1M, and 80mg of omeprazole. The blood samples are processed for blood count, biochemical and coagulation parameters, venous blood gases, blood group and serological tests.
- (5)
After 15min in wait of legal authorization, or less if such permission arrives earlier, the activities are conditioned to the purpose of possible donation. Based on the suitability criteria of the donor, the transplant coordinator decides the strategy to be followed (Table 3):
- (a)
Lung donation only (up until July 2013): following confirmation of death, a chest drain is quickly placed in either side of the thorax (anterior second intercostal space), mechanical ventilation is stopped, and the drains are filled with Perfadex® at a temperature of 4°C. The orotracheal tube is left open to the exterior.
- (b)
Kidney donation only (from July 2013): cannulation of the femoral vein and artery is carried out by the cardiovascular surgery team, with connection to the extracorporeal membrane oxygenation system (ECMO). At the time of connection to ECMO, cardiac compression and the respirator are disconnected. A Fogarty catheter is placed in the contralateral femoral artery to isolate the thoracic aorta from the abdominal aorta.
- (c)
Kidney and lung donation (from July 2013): the donor is connected to ECMO in the same way as above, followed by disconnection of the respirator, and a chest drain is quickly placed in either side of the thorax (anterior second intercostal space). The drains are filled with Perfadex® at a temperature of 4°C. The orotracheal tube is left open to the exterior.
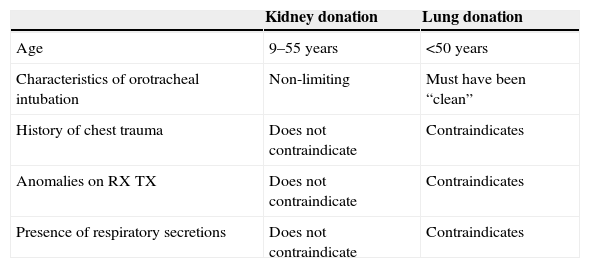
Table 3.Evaluation of possible kidney or lung donation.
Kidney donation Lung donation Age 9–55 years <50 years Characteristics of orotracheal intubation Non-limiting Must have been “clean” History of chest trauma Does not contraindicate Contraindicates Anomalies on RX TX Does not contraindicate Contraindicates Presence of respiratory secretions Does not contraindicate Contraindicates RX TX, chest X-rays.
- (a)
- (6)
Nasogastric and bladder catheterization. Biological samples are collected (urine, gastric juice and blood) for submission to the legal court authorities.
- (7)
End of the process in the ICU. The donor is left without cardiac compression and with the orotracheal tube open to the exterior.
Our program contemplates abdominal preservation under normothermal conditions (36°C) in the case of kidney donation only (nECMO); however, if donation is thoracic and abdominal, abdominal preservation requires hypothermia (4°C).
At the same time, the family of the possible donor is located and informed of the fatal outcome and of the possibility of organ donation. The relatives can see the patient in the ICU if they so wish. The donor is then moved to the operating room for organ harvesting.
The program involves continuous evaluation of the process. In this respect, if incidents occur or if the established time limits are exceeded (Fig. 1), the process is suspended and the patient is discarded as a donor.
The implementation of this non-heart beating donor program has been carried out in abidance with the principles of the non-heart beating donation consensus document.6 Spanish law protects, endorses and gives full coverage to the development of such programs.7
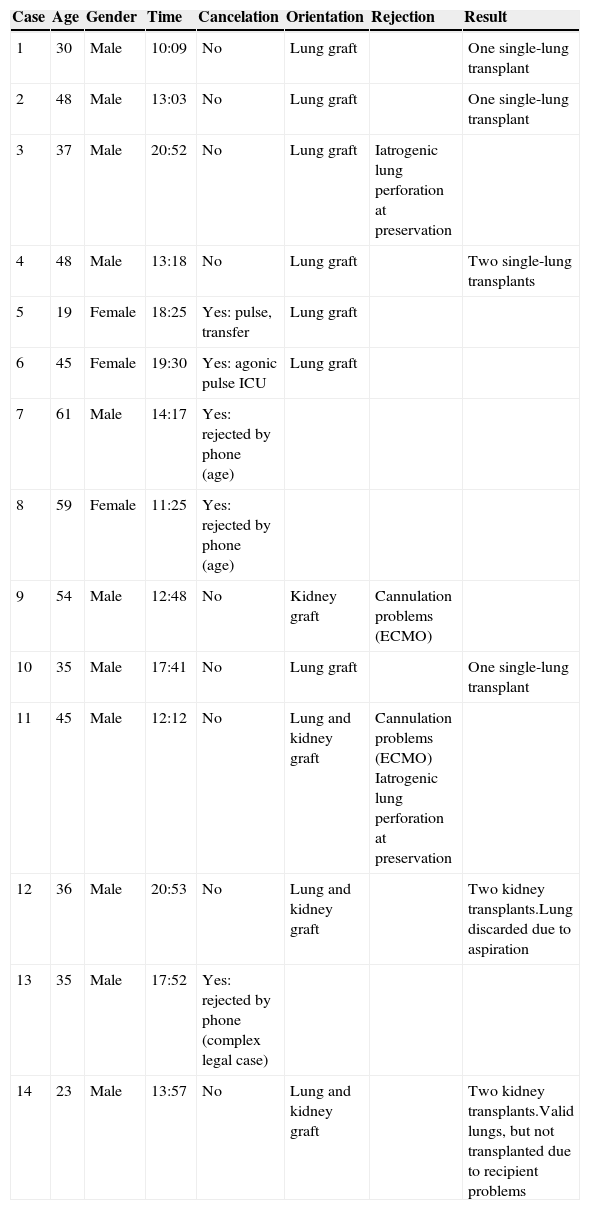
ResultsFourteen asystole code activations (i.e., 14 potential donors) were recorded during these first 14 months of the Maastricht type II non-heart beating donation program, with the transfer to hospital of 11 potential donors. Three potential donors were initially discarded due to failure to meet the activation criteria: donor age above the maximum limit in two cases, and legal complications in another. Table 4 describes the characteristics of the potential donors included in the series.
Characteristics of the patients included in the series.
| Case | Age | Gender | Time | Cancelation | Orientation | Rejection | Result |
|---|---|---|---|---|---|---|---|
| 1 | 30 | Male | 10:09 | No | Lung graft | One single-lung transplant | |
| 2 | 48 | Male | 13:03 | No | Lung graft | One single-lung transplant | |
| 3 | 37 | Male | 20:52 | No | Lung graft | Iatrogenic lung perforation at preservation | |
| 4 | 48 | Male | 13:18 | No | Lung graft | Two single-lung transplants | |
| 5 | 19 | Female | 18:25 | Yes: pulse, transfer | Lung graft | ||
| 6 | 45 | Female | 19:30 | Yes: agonic pulse ICU | Lung graft | ||
| 7 | 61 | Male | 14:17 | Yes: rejected by phone (age) | |||
| 8 | 59 | Female | 11:25 | Yes: rejected by phone (age) | |||
| 9 | 54 | Male | 12:48 | No | Kidney graft | Cannulation problems (ECMO) | |
| 10 | 35 | Male | 17:41 | No | Lung graft | One single-lung transplant | |
| 11 | 45 | Male | 12:12 | No | Lung and kidney graft | Cannulation problems (ECMO) Iatrogenic lung perforation at preservation | |
| 12 | 36 | Male | 20:53 | No | Lung and kidney graft | Two kidney transplants.Lung discarded due to aspiration | |
| 13 | 35 | Male | 17:52 | Yes: rejected by phone (complex legal case) | |||
| 14 | 23 | Male | 13:57 | No | Lung and kidney graft | Two kidney transplants.Valid lungs, but not transplanted due to recipient problems |
All asystole code activations occurred between 8 in the morning and 10 at night.
Most of the 14 potential donors (78%) were males. The median age was 41 years (IQR 33.7–48), and in 67.7% of the cases cardiorespiratory arrest occurred on the street.
The 11 eligible donors had a median age of 39.5 years (IQR 32–48), and all were males. Lung donation only was decided in 7 cases, kidney donation only was decided in one case, and both lung and kidney donation was decided in three cases (the decisions were made at out-hospital level). Of the 11 eligible donors, two were discarded upon arrival in the ICU due to a persistent pulse. The cardiopulmonary resuscitation protocols were continued in these cases.
In relation to the remaining 9 donors, iatrogenic organ damage during preservation (lung perforation or ECMO cannulation problems) precluded organ transplantation in two cases.
Finally, of the 7 effective donors, 5 corresponded to lung donation with 5 single-lung transplants (two performed in our center and three in La Coruña Hospital Complex), while two were kidney donors with four renal transplants (all in our center).
In the 7 effective donors some grafts were discarded for transplantation despite initial consideration for lung and kidney donation. Lung grafting was discarded in two cases due to technical problems during placement of the chest drains secondary to iatrogenic lung perforation, while another graft was discarded due to bronchopulmonary aspiration evidenced in the ICU, and in one donor the lungs were removed but finally not implanted due to problems with the recipient patient.
The reasons for discarding initially contemplated kidney donation were technical problems related to vascular cannulation for preservation with ECMO (two cases). In addition to the harvesting of solid organs, tissue and corneas could be obtained (with a total of 18 corneal grafts).
Eleven family interviews were held, and none of the families rejected donation. Likewise, there were no legal or forensic incidents.
The median time from cardiac arrest to renal preservation (with ECMO or nECMO) was 80min (IQR: 71–89), while the median time from cardiac arrest to lung preservation was 84min (IQR: 77–94). These times are clearly shorter than those reported by other groups.
The median in-hospital time from arrival in the ICU to only lung preservation was 27min (range 17–35), while the in-hospital time from arrival in the ICU to ECMO was 37min (range 29–45).
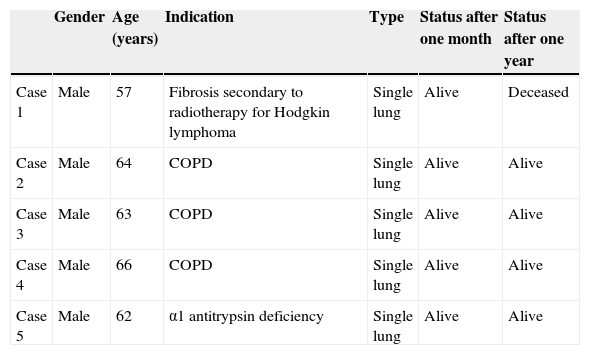
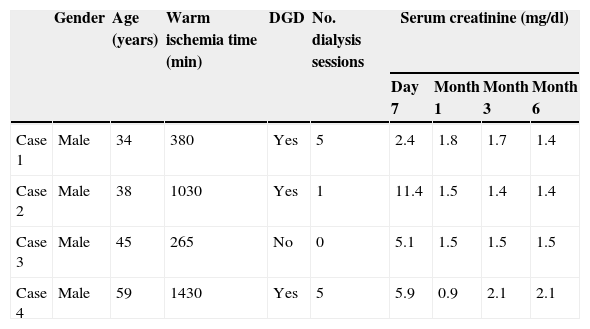
Tables 5 and 6 report the characteristics of the lung and renal recipients.
Characteristics of the lung transplant patients.
| Gender | Age (years) | Indication | Type | Status after one month | Status after one year | |
|---|---|---|---|---|---|---|
| Case 1 | Male | 57 | Fibrosis secondary to radiotherapy for Hodgkin lymphoma | Single lung | Alive | Deceased |
| Case 2 | Male | 64 | COPD | Single lung | Alive | Alive |
| Case 3 | Male | 63 | COPD | Single lung | Alive | Alive |
| Case 4 | Male | 66 | COPD | Single lung | Alive | Alive |
| Case 5 | Male | 62 | α1 antitrypsin deficiency | Single lung | Alive | Alive |
COPD, chronic obstructive pulmonary disease.
Characteristics of the kidney transplant patients.
| Gender | Age (years) | Warm ischemia time (min) | DGD | No. dialysis sessions | Serum creatinine (mg/dl) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Day 7 | Month 1 | Month 3 | Month 6 | ||||||
| Case 1 | Male | 34 | 380 | Yes | 5 | 2.4 | 1.8 | 1.7 | 1.4 |
| Case 2 | Male | 38 | 1030 | Yes | 1 | 11.4 | 1.5 | 1.4 | 1.4 |
| Case 3 | Male | 45 | 265 | No | 0 | 5.1 | 1.5 | 1.5 | 1.5 |
| Case 4 | Male | 59 | 1430 | Yes | 5 | 5.9 | 0.9 | 2.1 | 2.1 |
DGD, delayed graft dysfunction; m, months; min, minutes; RT, radiotherapy.
The results presented in this study confirm that a non-controlled cardiac death (Maastricht type II) donation program in a medium-sized city is feasible in application to both abdominal organs and lung grafts. Similar initiatives have been adopted elsewhere in Spain. As an example, Virgen de la Nieves Hospital started a Maastricht type II donation program, though covering a larger population and focused on abdominal organ donation.8 The salient feature that distinguishes our program from the rest of national or international donation programs is that both the diagnosis of death and abdominal and thoracic organ preservation are entirely carried out in the ICU.
In our experience, this distinguishing feature offers important benefits. In effect, centralization of all the processes avoids in-hospital transfers that prove very difficult in multi-monitored patients or cadavers subjected to mechanical cardiac compression, reducing the warm ischemia time of the organ and thus improving the recipient prognosis. In effect, the warm ischemia times in our program are notoriously shorter than those published in other series,9 and are fundamentally attributable to a very important shortening of the in-hospital phase, which is the phase that can be reduced through good management of the human and material resources.
Another advantage of our program is that attention to the family of a deceased person of this kind is more natural in the ICU than in an operating room. The ICU is a place where the family understands the extreme seriousness of the situation, facilitating understanding of what has happened, and predisposing to the acceptance of organ donation once the fatal outcome has been assimilated. Furthermore, the ICU avoids the need for transfer and the changing of clothing required when entering an operating room. In our experience none of the families refused organ donation.
An additional positive aspect of centralizing the entire process in the ICU is that in the event the transplant coordinator is unable to attend a call, the intensivist can initially act as coordinator, since he or she is always physically present and knows the process. This aspect is very important in small cities, where it is very difficult to gain experience with these procedures, since they are used only on an occasional basis.
Intensivist support of the entire process is reflected in lung preservation. The lung transplant team in our center is localized, and it was decided that lung preservation should be performed by whoever happens to be present, i.e., if the chest surgeon is present, preservation is performed by both professionals (chest surgeon and intensivist), while if the chest surgeon is unable to arrive on time, the process is performed by the intensivist on duty (staff member and resident or the transplant coordinator in person). There is no egocentricity in this procedure capable of slowing the process. Likewise, the fact that the transplant coordinator is an intensivist belonging to the center facilitates integration of the entire process.
Expansion of the program to include kidney harvesting did not pose problems, since the ECMO program (both venous–venous and arteriovenous) was fully implemented in our center, and it is commonly used in the ICU in application to pneumological patients, as a bridge to heart or lung transplantation, or in the postoperative period of such transplant procedures.10,11
One of the most controversial aspects of introducing a Maastricht type II non-heart beating donation program is the minimum population size required to guarantee the viability of the program. A threshold population of 500,000 inhabitants was established, though experiences in slightly smaller populations have yielded promising results, as for example in Granada.8 Obviously, the larger the population, the larger the number of potential donors. However, our initial activity shows that it is feasible to obtain 8–10 donations a year, which in our Autonomous Community would mean 13–16 donors per million inhabitants. Another aspect that would help improve the potential of the program is to increase the contemplated age limit to 60 years. Furthermore, equipping all mobile ICU ambulances attending the population outside the city of Santander with mechanical cardiac compressors would also increase the population area.
A peculiarity of donation of this kind in a small city is the fewer number of non-sanitary professionals forming part of the program. In this regard, there are only 6 courts of first instance in Santander; as a result, legal authorization for organ preservation and harvesting must be provided by one of the 6 judges. Before starting our program, each of the court of first instance judges and the senior judge were personally informed of the characteristics of the program, in the same way as the forensic physicians in our Autonomous Community. The forensic specialist and judge on duty are informed by the transplant coordinator of the existence of a potential non-heart beating donor while the latter is being transferred to the hospital center. This closeness to the legal professionals facilitates collaboration and contributes to reduce court denials. In our program we have had no such denials referred to organ preservation and/or extraction. Likewise, all permissions for extraction were granted in under 5min, without the 15min of administrative silence contemplated by current legislation. This fact contributes to shorten the warm ischemia time even further.
The scarcity of organs is a problem faced by all transplant programs, though it is particularly prevalent in lung transplantation, since lungs are harvested from only 15% of all organ donors.12,13 For this reason, specific multiorgan donor management strategies,14,15 the use of elderly donors,16,17 or non-heart beating donation18,19 are crucial measures for increasing the pool of available grafts.20
The functional outcomes of the transplanted patients are very good, despite the learning curve all of our team has had to complete in relation to the preservation of organs from non-heart beating donors. All the lung transplant patients are alive, except the first patient, with survival rates comparable to those of the national series.4,21 Likewise, it must be noted that the 5 recipients were all very elderly (Table 5) and with greater than usual morbidities. This is in contrast to the mean age of the lung transplant recipients in Spain (48.2 years, standard deviation 14.7)21 and our own local series of lung transplant cases.22
In the case of the renal graft recipients the prognosis was likewise excellent (Table 6): all grafts are functioning normally and with good creatinine levels.
We consider that there is important room for improvement in our program, since we have had a number of lung iatrogenic problems in the form of lung perforation and ECMO cannulation difficulties resulting from the urgent need to shorten the ischemia times. These technical shortcomings, associated to the learning curve, have adversely affected the number of available grafts (Table 4).
The potential of non-heart beating donation in Spain has not been established, and experiences such as those in Granada, Alicante and our own speak in favor of centers located in areas with a smaller population. Each program must adapt to its existing possibilities, though in all cases correct coordination of all the implicated teams is required in order to cope with the technical and organizational difficulties associated with the implementation of programs such as these. This logistic complexity of Maastricht type II donation programs implies the development of new strategies designed to reduce such complexity. In this context, initiatives such as our own highlight the role of the ICU in such activities and the possibilities of medium-sized cities, since the warm ischemia times are shortened by avoiding transfers between Departments within the hospital, and the distances involved are shorter–thereby reducing the out-hospital times.
This study, and our non-heart beating donation program, has a series of limitations, such as the very limited sample size and the difficulty of ensuring the necessary experience for all the intervening parties. Likewise, it is difficult to start a non-heart beating lung donation program in centers without lung transplantation facilities, given the short ischemia time tolerated by the lung grafts. Furthermore, it is not clear which method is best for combining abdominal preservation–where normothermia appears to be the most recommended strategy–and pulmonary preservation, where hypothermia is required.
ConclusionsA Maastricht type II program in a small city is viable for both abdominal and thoracic organs. The start of the program has been very cautious, and its potential can be easily improved by increasing the contemplated age limit to 60 years and equipping all mobile ICU ambulances with mechanical cardiac compressors. The development of the entire process encompassing the diagnosis of death, graft preservation and family interview in the ICU–avoiding emergency care and operating rooms–shortens the warm ischemia times and improves the post-transplantation outcomes.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Thanks are due to the lung transplant team, renal transplant team, Department of Cardiovascular Surgery, and the nursing and perfusionist team of Marqués de Valdecilla University Hospital, for their enthusiastic support of this organ donation program.
Please cite this article as: Miñambres E, Suberviola B, Guerra C, Lavid N, Lassalle M, González-Castro A, et al. Experiencia de un programa de donación en asistolia Maastricht II en una ciudad de pequeño tamaño: resultados preliminares. Med Intensiva. 2015;39:433–441.