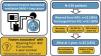
Unplanned invasive mechanical ventilation (IMV) is associated with high mortality in lung cancer patients. We aimed to identify factors associated with weaning from IMV, intensive care unit (ICU) survival and 1-year survival in lung cancer patients requiring unplanned IMV.
DesignRetrospective observational study (2007–2017).
SettingUniversity-affiliated ICU.
PatientsLung cancer patients requiring unplanned IMV.
InterventionNone.
Main variables of interestWeaning from IMV, ICU and 1-year survival.
ResultsOf the 136 patients included in the analysis (age 64 (9) years, male 110 (81%), metastatic disease 97 (62%)), 52 (38%) were weaned from IMV, 51 (38%) were discharged from ICU and 22 (16%) were alive at 1year. The main indication for intubation was acute respiratory failure. In multivariate analysis, PaO2/FiO2 >175mmHg at ICU admission and intubation before ICU admission were associated with successful weaning from IMV while intubation for cardiac arrest was associated with weaning failure. Same factors were associated with ICU survival. Absence of metastasis at ICU admission and lung resection surgery were independently associated with 1-year survival.
ConclusionsA significant proportion of patients with lung cancer treated with unplanned IMV could be weaned from IMV and survived to ICU discharge, especially in the absence of severe hypoxemia at ICU admission. The low one-year survival was mostly driven by metastatic status.
La ventilación mecánica invasiva (VMI) no planificada se asocia a una elevada mortalidad en pacientes con cáncer de pulmón. El objetivo de nuestro estudio fue identificar los factores asociados con el destete de la VMI y la supervivencia en unidad de cuidados intensivos (UCI) y a 1 año en pacientes con cáncer de pulmón que requieren VMI no planificada.
DiseñoEstudio observacional retrospectivo (2007–2017).
ÁmbitoUCI universitaria.
PacientesPacientes con cáncer de pulmón que requirieron VMI no planificada.
IntervenciónNinguna.
Variables de interés principalesDestete de la VMI y supervivencia a 1 año.
ResultadosSe incluyeron 136 pacientes: edad 64(9) años, sexo masculino 110 (81%), enfermedad metastásica 97 (62%). De ellos, 52 (38%) fueron destetados de la VMI. La supervivencia en UCI y al año era 38% (n=51) y 16% (n=22) respectivamente. La principal indicación de intubación fue la insuficiencia respiratoria aguda. En el análisis multivariante, la PaO2/FiO2 >175mmHg al ingreso en la UCI y la intubación realizada antes del ingreso en la UCI se asociaron a un destete satisfactorio de la VMI, mientras que la intubación por parada cardiaca se asoció a un fracaso del destete. Los mismos factores se encontraron para la supervivencia en UCI. La ausencia de metástasis al ingreso en la UCI y la cirugía de resección pulmonar se asociaron de forma independiente con la supervivencia a 1 año.
ConclusionesUna proporción significativa de pacientes con cáncer de pulmón tratados con VMI no planificada pueden ser destetados de la VMI, especialmente en ausencia de hipoxemia grave al ingreso en UCI. La baja supervivencia a un año se debió principalmente al estado metastásico.
Lung cancer is the second most common cancer worldwide and remains the leading cause of cancer-related deaths, with nearly 2 million deaths each year.1 Admissions of lung cancer patients to the intensive care unit (ICU) are frequent, and 20–80% of these patients are treated with invasive mechanical ventilation (IMV), which is associated with a very high short-term mortality rate.2–6 Unplanned intubation in patients with lung cancer occurs in emergency situations and frequently occurs before ICU admission. The patient’s wishes regarding intensive care and oncologic prognosis are often unknown at the time of intubation.7 Thus, the level of care and therapeutic goals should be discussed as soon as possible after the initiation of IMV. Even in cases where short- or medium-term prognosis is poor because of cancer, weaning from IMV is an acceptable therapeutic goal. Indeed, removal of the endotracheal tube can improve patient comfort. In the most favorable cases, patients are discharged from the ICU and continue oncological treatments.2,4,5,8 However, most reports indicate that the weaning process fails in a majority of these patients.2,5,6,9,10 Thus, ICU care is often considered as “futile” in this setting.11 Therefore, it is important to identify the factors associated with successful weaning from IMV and long-term survival in patients with lung cancer requiring unplanned intubation. This would help to provide adequate prognostic information to patients or relatives and/or to prevent unnecessary life-prolonging therapy in ICU. The primary endpoint was to identify factors associated with weaning success in lung cancer patient requiring unplanned IMV. We also aimed to identify the independent factors associated with ICU survival and 1-year survival.
Patients and methodsStudy designWe conducted a retrospective cohort study of patients with lung cancer requiring unplanned IMV from 2007 to 2017. The study received approval from the regional ethics committee with a waiver for written informed consent because of the retrospective nature of the study. The study was conducted in compliance with the ethical standards detailed in the 1964 Declaration of Helsinki. Non-opposition for the use of anonymous data for research purposes was obtained during the ICU stay.
PatientsAll adult patients with active lung cancer or in remission for less than 5 years requiring unplanned IMV and admitted to our 15-bed university-affiliated medical ICU were included.
Data collectionThe following variables were collected for each patient: age, gender, comorbidities, body mass index (BMI), source of admission (home or hospital), Charlson Comorbidity Index (CCI),12 and performance status13 assessed in the week prior to ICU admission.
The following lung cancer features were recorded: histological type, follow-up in an oncologic reference center, metastatic disease as defined in the 8th version of TNM classification,14 targetable driver mutation (EGFR, ALK, ROS1), time since cancer diagnostic, anticancer treatment before ICU admission. The cancer status was determined for each patient at the time of admission and classified as follows: newly diagnosed (treatment not yet started), newly treated (treatment started for less than 2 months or without oncologic re-evaluation), progression/recurrence, remission (for less than 5 years).
The main indication for IMV and the place of intubation were collected. Simplified Acute physiology Score II (SAPS II) was recorded15 as well as Sequential Organ failure Assessment (SOFA) score evaluated at 24 and 72h from admission.16 The ratio of partial oxygen pressure to oxygen inspired fraction (PaO2/FiO2) was used to describe the severity of acute respiratory failure (ARF) at ICU admission (in the case of several values, the worst was considered). Acute respiratory distress syndrome (ARDS) was defined according to the Berlin definition.17
The use of non-invasive ventilation (NIV) before intubation and the duration of IMV were recorded. Weaning failure was defined as the need for reintubation or death within 48h following weaning from IMV.18 Other interventions were collected during the ICU stay including paralyzing agents, prone positioning, renal replacement therapy, use of vasopressors and anti-cancer chemotherapy in ICU. End-of-life decisions were recorded. Continuation of cancer treatment after ICU discharge, hospital discharge, survival at 6 months and at 1year were also collected.
Statistical analysisValues are presented as mean (standard deviation, SD) or number (%), as appropriate. Univariate comparisons were performed using MannWhitney U test for continuous variables and Fisher’s exact test for categorical variables, as appropriate. Backward stepwise multivariate analysis using a logistic regression model was performed to assess the factors predicting successful extubation and survival in ICU. Following univariate analysis, clinically relevant variables with p≤0.10 were included in the model. Potential confounding factors were eliminated if p>0.10. Odds ratios (OR) were estimated with 95% confidence intervals (95% CI). When needed, quantitative variables were transformed into dummy variables according to their median. Because of the collinearity with PaO2/FiO2 ratio at admission, the SAPS II, SOFA score, and ARDS variables were not included in the model. Also, because targetable driver mutations were sought in a limited number of patients, this variable was not included in the model.
The independent contribution to mortality by parameters possibly influencing the 1-year outcome was tested by a backward stepwise multivariate Cox proportional hazard model, using time to death as the dependent variable. In addition, we introduced age in the model to avoid retaining confounding factors related to this major demographic covariate. Hazard ratios (HR) were estimated with 95% CI.
To assess the potential influence of time, patient characteristics and outcomes were compared between 2 consecutive periods: 2007–2011, 2012–2017.
GraphPad Prism 9.0 software (GraphPad Software, CA, USA) and WizardPro (version 1.9 29) were used to perform statistical analysis. A p value <0.05 value was considered statistically significant.
ResultsPopulationOne hundred thirty-six patients were included in this study (Table 1). Half of the patients were followed up in an oncologic reference center, and two-thirds had metastatic disease (Table 1). The most common histological type of cancer was adenocarcinoma (46%). A targetable driver mutation was present in 5 patients, including 4 EGFR-activating mutations and 1 ALK rearrangement. Eleven patients were treated with targeted therapies (erlotinib (n=9), osimertinib (n=1), and crizotinib (n=1)), and three received nivolumab.
Baseline characteristics.
| All(n=136) | Weaned(n=52) | Unweaned(n=84) | p | |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 64 (9) | 63 (9) | 64 (9) | 0.40 |
| Male gender | 110 (81%) | 41 (79%) | 69 (82%) | 0.80 |
| Body mass index, kgm−2 | 24 (5) | 23 (4) | 24 (5) | 0.14 |
| Comorbidities | ||||
| COPD | 60 (44%) | 26 (50%) | 34 (40%) | 0.36 |
| Coronary artery disease | 28 (21%) | 14 (27%) | 14 (17%) | 0.22 |
| Diabetes mellitus | 34 (25%) | 13 (25%) | 21 (25%) | >0.99 |
| Chronic renal failure | 8 (6%) | 7 (13%) | 1 (1%) | <0.01 |
| Charlson Comorbidity Index | 8 (3) | 8 (3) | 8 (2) | 0.45 |
| Performance status | 0.01 | |||
| 0−1 | 70 (51%) | 34 (65%) | 36 (43%) | |
| 2 or more | 65 (48%) | 18 (35%) | 48 (57%) | |
| Lung cancer | ||||
| Follow-up in reference center | 70 (51%) | 29 (56%) | 41 (49%) | 0.48 |
| Time since cancer diagnosis, days | 271 (432) | 344 (548) | 225 (336) | 0.23 |
| Time since last treatment, days | 115 (265) | 160 (331) | 82 (204) | 0.59 |
| Cancer histological type | 0.51 | |||
| Adenocarcinoma | 63 (46%) | 24 (46%) | 39 (46%) | |
| Squamous cell carcinoma | 26 (19%) | 9 (17%) | 17 (20%) | |
| Small cell lung cancer | 22 (16%) | 11 (21%) | 11 (13%) | |
| Other | 18 (13%) | 6 (11%) | 12 (14%) | |
| Unknown | 7 (5%) | 2 (4%) | 5 (6%) | |
| Metastatic disease | 92 (67%) | 31(60%) | 61 (72%) | 0.13 |
| Targetable driver mutation | 5 (4%) | 4 (8%) | 1 (1%) | 0.07 |
| Anticancer treatment | ||||
| Chemotherapy/targeted therapy | 76 (56%) | 29 (56%) | 47 (56%) | >0.99 |
| Lung resection | 21 (15%) | 12 (23%) | 9 (11%) | 0.09 |
| Radiation | 39 (29%) | 15 (29%) | 24 (29%) | >0.99 |
| Not yet started | 44 (32%) | 16 (31%) | 28 (33%) | 0.85 |
| Number of treatment lines | 0.69 | |||
| 0−1 | 105 (77%) | 42 (81%) | 63 (75%) | |
| 2 or more | 31 (23%) | 10 (19%) | 21 (25%) | |
| Cancer status | 0.61 | |||
| Remission | 47 (35%) | 21 (40%) | 26 (31%) | |
| Newly diagnosed | 44 (32%) | 16 (31%) | 28 (33%) | |
| Newly treated | 12 (9%) | 5 (10%) | 7 (8%) | |
| Recurrence / Progression | 33 (24%) | 10 (19%) | 23 (27%) |
Data are expressed as mean (SD) or number (%).
COPD: Chronic obstructive pulmonary disease.
In most cases, the patients were admitted to the ICU from their homes or via the emergency room (Table 2). The main indication for intubation was ARF, mainly due to pneumonia (including 6 pneumocystis pneumoniae), followed by cancer-related complications (12 tracheobronchial obstructions and atelectasis, 5 pulmonary lymphangitic carcinomatosis, 5 hemoptysis, 4 pleural effusions, 1 superior vena cava syndrome, 1 radiation-induced lung injury, and 1 tumor lysis syndrome). Intubation was performed before ICU admission in more than 50% of cases (Table 2). Among the 112 (78%) patients with ARDS, neuromuscular blocking agents were used in 22 (26%) and prone positioning in 4 (3%). Most patients had extrapulmonary organ failure and received vasopressors.
Organ failure and treatments in ICU.
| All(n=136) | Weaned(n=52) | Unweaned(n=84) | p | |
|---|---|---|---|---|
| Source of admission | 0.86 | |||
| Home/Emergency room | 76 (56%) | 30 (57%) | 46 (54%) | |
| General hospital | 60 (44%) | 22 (42%) | 38 (45%) | |
| Indication for intubation | ||||
| Pulmonary sepsis | 59 (43%) | 23 (44%) | 36 (43%) | >0.99 |
| Cancer related events | 30 (22%) | 8 (15%) | 22 (26%) | 0.20 |
| Cardiac arrest | 16 (12%) | 1 (2%) | 15 (18%) | <0.01 |
| Neurological failure | 15 (11%) | 11 (21%) | 4 (5%) | <0.01 |
| Non-respiratory sepsis | 7 (5%) | 3 (6%) | 4 (5%) | >0.99 |
| Surgical adverse events | 7 (5%) | 4 (8%) | 3 (4%) | 0.43 |
| Cardiogenic pulmonary edema | 2 (1%) | 2 (4%) | 0 (0%) | 0.14 |
| SAPS II | 61 (21) | 58 (15) | 64 (24) | 0.30 |
| SOFA score at ICU admission | ||||
| Respiratory | 2.8 (1.1) | 2.2 (1.1) | 2.8 (0.9) | <0.01 |
| Cardiovascular | 2.9 (1.7) | 2.8 (1.8) | 3.0 (1.7) | 0.32 |
| Renal | 0.6 (1.0) | 0.5 (0.9) | 0.6 (1.0) | 0.59 |
| Coagulation | 0.4 (0.9) | 0.2 (0.6) | 0.5 (1.0) | 0.03 |
| Hepatic | 0.1 (0.5) | 0.1 (0.3) | 0.1 (0.6) | 0.76 |
| Neurological | 1.3 (1.6) | 1.3 (1.6) | 1.3 (1.6) | 0.86 |
| Total | 7.8 (3.1) | 7.0 (2.6) | 8.3 (3.3) | 0.02 |
| Acute respiratory failure | ||||
| PaO2/FiO2 at admission, mmHg | 195 (105) | 240 (119) | 168 (85) | <0.01 |
| ARDS at inclusion | 112 (82%) | 37 (71%) | 75 (89%) | 0.01 |
| ICU treatments | ||||
| Invasive mechanical ventilation | ||||
| Started before ICU admission | 76 (56%) | 35 (67%) | 41 (56%) | 0.05 |
| Started after NIV failure | 34 (25%) | 13 (25%) | 21 (25%) | 0.13 |
| Duration, days | 7.8 (8.0) | 7.4 (7.5) | 8.1 (8.3) | 0.90 |
| Renal replacement therapy | 10 (7%) | 2 (4%) | 8 (10%) | 0.37 |
| Vasopressors | 106 (78%) | 38 (73%) | 68 (81%) | 0.29 |
| Chemotherapy | 8 (6%) | 3 (6%) | 5 (6%) | 0.99 |
Data are expressed as mean (SD) or number (%).
SAPS II: Simplified Acute Physiology Score II; SOFA: Sequential Organ Failure Assessment; ICU: Intensive care unit; PaO2/FiO2: arterial partial pressure of oxygen to fraction of inspired oxygen; ARDS: Acute respiratory distress syndrome; NIV: noninvasive ventilation.
Fifty-two patients (38%) were successfully weaned from IMV (Tables 1 and 2). All these patients except one were discharged alive from ICU. Weaning form IMV success rate did not differ between the 2 predefined periods of the study (p=NS). The proportion of patients with chronic renal failure, with performance status at 0−1, intubated prior to admission, and intubated for neurological failure, as well as the SOFA score and PaO2/FiO2 ratio, were significantly different in patients who were weaned from IMV or not (Tables 1 and 2). None of the 6 patients diagnosed with pneumocystis could be weaned from IMV.
In multivariate analysis, PaO2/FiO2 >175mmHg (i.e., median value of the cohort) at ICU admission and intubation before ICU admission were associated with successful weaning from IMV while cardiac arrest was associated with weaning failure (Table 3). The factors associated with survival in the ICU were the same as those associated with weaning from IMV (Table 3).
Factors independently associated with successful weaning from invasive mechanical ventilation and ICU survival.
| Odds ratio | 95% CI | p | |
|---|---|---|---|
| Weaning from IMV | |||
| Cardiac arrest | 0.045 | 0.005−0.376 | 0.004 |
| IMV started before ICU admission | 2.347 | 1.052−5237 | 0.037 |
| PaO2/FiO2 at admission >175mmHg | 3.704 | 1.674−8196 | <0.001 |
| ICU survival | |||
| Cardiac arrest | 0.048 | 0.006−0.401 | 0.005 |
| IMV started before ICU admission | 2.279 | 1.021−5086 | 0.044 |
| PaO2/FiO2 at admission >175mmHg | 3.354 | 1.515−7426 | 0.003 |
CI confidence interval; IMV: invasive mechanical ventilation; ICU: intensive care unit; PaO2/FiO2: arterial partial pressure of oxygen to fraction of inspired oxygen.
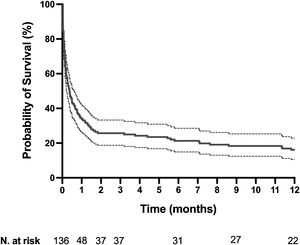
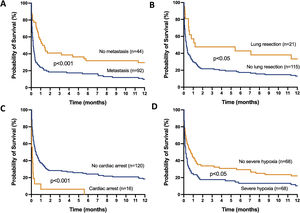
The mortality rates in the ICU, hospital, and 6 months and 1year after ICU admission were 62%, 71%, 79%, and 84%, respectively. One-year mortality rate was not significantly different between the 2 predefined periods of the study (p=NS). Most deaths occurred within 1 month after ICU admission (Fig. 1). End-of-life decisions were reported in 75 (55%) patients, on average 6 (8) days after ICU admission. These decisions were significantly more frequent (p<0.01) in the unweaned group than in the weaned group (66/82 (80%) vs. 9/54 (17%]). The factors associated with one-year mortality after ICU admission are summarized in Table 4. Cumulative mortality at 1year was significantly (p<0.05 by log-rank test) higher in patients who had metastasis, no lung resection surgery, cardiac arrest and low PaO2/FiO2 ratios (Fig. 2). In multivariate analysis, absence of metastasis at ICU admission (HR 0.57, 95%CI [0.39−0.82]; p<0.01]) and lung resection surgery (HR 0.62, 95%CI [0.38−0.99]; p<0.05]) were independently associated with 1-year mortality rate.
Characteristics of survivors and non-survivors at 1year after ICU admission.
| Survivors(n=22) | Non-survivors(n=114) | p | |
|---|---|---|---|
| Demographics | |||
| Age, years | 62 (9) | 64 (9) | 0.96 |
| Male gender | 15 (68%) | 95 (83%) | 0.17 |
| Body mass index, kgm−2 | 24 (4) | 23 (5) | 0.85 |
| Performans status 0 or 1 | 15 (68%) | 55 (48%) | 0.22 |
| Lung cancer | |||
| Time since cancer diagnosis, days | 444 (743) | 237 (336) | 0.10 |
| Cancer histological type | 0.91 | ||
| Non-small cell lung cancer | 16 (73%) | 91 (80%) | |
| Small cell lung cancer | 4 (18%) | 18 (16%) | |
| Unknown | 2 (9%) | 5 (4%) | |
| Metastatic disease | 9 (41%) | 83 (73%) | < 0.01 |
| Targetable driver mutation | 3 (14%) | 2 (2%) | 0.03 |
| Lung resection | 7 (32%) | 14 (12%) | 0.045 |
| Treatment lines <2 | 20 (91%) | 85 (75%) | 0.61 |
| Cancer status | 0.33 | ||
| Remission | 10 (45%) | 37 (32%) | |
| Active | 12 (55%) | 77 (68%) | |
| Indication for intubation | |||
| Pulmonary sepsis | 10(45%) | 49 (43%) | >0.99 |
| Cancer related events | 4 (18%) | 26 (23%) | 0.78 |
| Cardiac arrest | 0 (0%) | 16 (14%) | 0.07 |
| Neurological failure | 4 (18%) | 11 (10%) | 0.27 |
| Non-respiratory sepsis | 0 (0%) | 7 (6%) | 0.59 |
| Surgical adverse events | 3 (14%) | 4 (4%) | 0.08 |
| Cardiogenic pulmonary edema | 1 (5%) | 1 (1%) | 0.30 |
| SAPS II | 55 (17) | 62 (22) | 0.17 |
| SOFA score at admission | 6.8 (2.7) | 8.0 (3.2) | 0.86 |
| PaO2/FiO2 at admission, mmHg | 248 (135) | 185 (96) | 0.01 |
| ICU treatments | |||
| Renal replacement therapy | 1 (5%) | 9 (8%) | 0.92 |
| Vasoactive drugs | 14 (64%) | 81 (71%) | 0.61 |
| Chemotherapy | 2 (9%) | 6 (5%) | 0.84 |
Data are expressed as mean (SD) or number (%).
SAPS II: Simplified Acute Physiology Score II; SOFA: Sequential Organ Failure Assessment; PaO2/FiO2: arterial partial pressure of oxygen to fraction of inspired oxygen; ICU: Intensive care unit.
Kaplan-Meier plots showing representative factors influencing probability of survival.
Metastasis (A), absence of lung resection as a treatment for lung cancer (B), cardiac arrest as the reason for invasive mechanical ventilation (C) and severe hypoxemia (i.e., PaO2/FiO2 ≤175mmHg) at intensive care unit admission (D), were associated (p<0.05 by the log-rank test) with a significantly higher rate of mortality.
Emergency chemotherapy was performed in eight (6%) patients, all of whom had newly diagnosed small cell lung cancer. No targeted therapy was started in ICU. Of the 36 ICU survivors with planned cancer treatment, 23 (64%) received cancer treatment after ICU stay. For patients with a targetable driver mutation, one died in the ICU, one in the month following ICU admission, and three were still alive at 1year.
DiscussionThis study found that more than one-third of lung cancer patients with unplanned IMV could be weaned from the ventilator, and identified that patients who were not intubated before ICU admission, with severe hypoxemia, and/or with cardiac arrest were less likely to be weaned from IMV. None of the factors related to lung cancer independently influenced the weaning success. Conversely, one-year mortality was only driven by factors related to lung cancer (metastatic status and no lung cancer resection).
Data on weaning success in patients with lung cancer requiring unplanned IMV are scarce.9,10,19 In the present study, we found that 38% of patients were successfully weaned off the ventilator. This figure is much higher than the previously reported 15% (7/46) reported by Ewer et al. in 1986.10 Nevertheless, patients were at a higher risk of weaning failure, as they all had active lung cancers versus 65% in our study. A study published approximately 20 years later reported a closer success rate of extubation (27%) in 81 lung cancer patients treated with IMV for ARF.9 More recently, in a selected population of lung cancer patients with EGFR mutations treated with tyrosine kinase inhibitors as a first-line therapy, weaning from unplanned IMV was successful in twice as many patients as in our study.19 These latter results are in line with our own, since four of the five patients (80%) in our cohort treated with targeted therapy could be weaned from IMV. Nevertheless, these patients still represent a minority of those managed in the ICU.4
In the present study, the severity of hypoxemia at admission was the main factor associated with weaning from IMV. This result is consistent with those of previous studies on oncologic patients with or without lung cancer.4,10,19,20 The poor prognosis related to respiratory failure in patients with lung cancer could be explained by the fact that the causes of hypoxemia are, in many cases, poorly reversible (e.g., proximal tumoral airway obstruction, lymphangitic carcinomatosis, smoking-related pulmonary diseases). In our cohort, delayed IMV (from ICU admission) was also associated with a higher risk of weaning failure. In line with our findings, previous studies reported that late initiation of IMV or IMV initiation after NIV failure was a poor prognostic factor in cancer patients with ARF.20,21 Interestingly, in our study, weaning from IMV was not associated with cancer characteristics. Nevertheless, there was a limited number of patients with proximal airway obstruction, which is an important prognostic factor in other studies.2,22 Finally, we identified cardiac arrest as the cause of unplanned IMV as an independent factor for weaning failure. Given the very poor prognosis of patients with asphyxia-induced cardiac arrest in the general population,23 this finding is not surprising.
In the last two decades, the outcomes of oncological patients requiring intensive care have improved.10,19–21,24 Nevertheless, the 1-year mortality of patients with lung cancer admitted to the ICU and receiving or not receiving IMV remains as high as 70%.4 Early data on the mortality of lung cancer patients requiring unplanned IMV reported a mortality rate of approximately 90%.9,10 However, recent data are lacking. In the present study, we reported a slightly lower 1-year mortality rate (84%). Nevertheless, our cohort included patients with a better prognosis at baseline than those in previous studies,9,10 as it included patients in remission and IMV for causes other than respiratory failure. Apart from the severity of hypoxemia at ICU admission, the 2 factors independently associated with 1-year outcomes were related to lung cancer characteristics (metastatic status and lung cancer resection). This finding is consistent with prior studies showing that metastasis and/or cancer progression and the use of IMV for ARF are the most important predictive factors of poor long-term outcomes.4,21,22,25 Knowledge of these prognostic factors is important to discuss the level of therapeutic commitment in close collaboration with the oncologist, especially for patients with advanced disease.26,27 Another important element to consider is the possibility of resuming or continuing oncological treatment (when indicated) after ICU discharge. This was the case for more than half of the patients included in our study, suggesting that unplanned IMV only moderately compromises the continuation of essential oncological treatments. This finding confirms those of previous studies that included a large proportion of patients who did not receive IMV.5,8,28
This study has several limitations. First, this study analyzed data from patients admitted to the ICU over a long period of time (11years), ending in 2017, whereas the prognosis of cancer patients requiring ICU care improved during the study period. Nevertheless, the patient characteristics and outcomes did not change over time. This is in line with a recent study that showed no change in mortality between 2007 and 2018 in lung cancer patients admitted to the ICU for life-threatening complications.4 Second, there were a limited number of patients for whom targetable mutations were assessed, and only 11 (8%) patients received targeted therapy. Third, we included a heterogeneous population of patients with different cancer stages and long‑term prognosis.
In conclusion, our study highlights that a significant proportion of lung cancer patients treated with unplanned IMV can be weaned from IMV, especially in the absence of severe hypoxemia at ICU admission. Most eligible patients received further oncological treatment after ICU discharge. However, one-year mortality was very high and was mostly driven by metastatic status. These results would help provide adequate prognostic information to relatives and/or prevent unnecessary life-prolonging therapy in the ICU.
Authors’ contributionEC and MC participated in the acquisition, analysis and interpretation of data and drafted the manuscript. MC performed the statistical analyses. MS and RH participated in acquisition of data and were involved in revising the manuscript critically. LA participated in the design and coordination of the study, helped to draft the manuscript. All authors read and approved the final version of the manuscript.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors
Competing interestsAll the authors declare they have no competing interests.