Edited by: Ana Ochagavía - Hospital Universitario de Bellvitge. L'Hospitalet de Llobregat. Barcelona. Spain.
Last update: March 2024
More infoUltrasound is an essential diagnostic tool in critically ill patients with extracorporeal membrane oxygenation (ECMO). With it, we can make an anatomical and functional (cardiac, pulmonary and vascular) evaluation which allows us to execute an adequate configuration, guides implantation, helps clinical monitorization and detects complications, facilitates withdrawal and complete post-implant evaluation. In patients with ECMO as respiratory support (veno-venous), thoracic ultrasound allows monitoring pulmonary illness evolution and echocardiography the evaluation of biventricular function, especially right ventricle function, and cardiac output to optimize oxygen transport. In ECMO as circulatory support (veno-arterial), echocardiography is the guide of hemodynamic monitoring, allows detecting the most frequent complications and helps the weaning. In ECMO teams, for a proper management of these patients, there must be trained intensivists with advanced knowledge on this technique.
La ecografía es un instrumento diagnóstico fundamental en el paciente crítico con membrana de oxigenación extracorpórea (ECMO). Con ella podemos hacer una evaluación anatómica y funcional (cardiaca, pulmonar y vascular) para plantear una adecuada configuración; además, guía su implante, ayuda en la monitorización clínica y la detección de complicaciones, facilita su retirada y completa la evaluación postimplante. En los pacientes con ECMO como soporte respiratorio (veno-venosa), la ecografía torácica permite monitorizar la evolución de la enfermedad pulmonar y la ecocardiografía la evaluación de la función biventricular, especialmente la derecha, y el gasto cardiaco para optimizar el transporte de oxígeno. En la ECMO como soporte circulatorio (veno-arterial), la ecocardiografía supone la guía de la monitorización hemodinámica, permite detectar las principales complicaciones y ayuda al destete del dispositivo. En los equipos ECMO, para un adecuado manejo de estos pacientes, debe haber intensivistas entrenados y con conocimientos avanzados sobre esta técnica.
Extracorporeal membrane oxygenation (ECMO) is a mechanical support system used to secure total or partial cardiopulmonary stabilization in patients with circulatory and/or respiratory failure. Although the history of ECMO goes back over 50 years since it was first used for therapeutic purposes,1 it has only become widely used throughout the world in recent decades. The introduction of new devices and materials, the results obtained during the influenza A pandemic,2,3 and patient centralization in reference centers with multidisciplinary teams are a number of the factors that have caused ECMO to form part of the therapeutic measures for dealing with refractory cardiorespiratory failure. Due to the characteristics of these patients and the complexity of the processes, the available scientific evidence is limited,4,5 though many studies have evidenced the usefulness of the technique, and for this reason different scientific societies have established recommendations on its use.6–8
The use of ultrasound in critical patients is an established practice and is crucial for both the general and specific evaluation of cardiovascular and respiratory disease. Despite the complexity of the exploration and the technical requirements, it can afford information with a high diagnostic capacity at the patient’s bedside and on an immediate basis.9,10 In the case of patients with ECMO, and due to the increase in its indications and configurations, ultrasound – particularly transthoracic echocardiography (TTE) or transesophageal echocardiography (TEE) in the case of a poor exploration window – has become a key instrument.11,12 Adequately trained professionals are needed, since ultrasound allows precise anatomical and functional cardiopulmonary evaluation, guides the insertion and placement of cannulas, helps to optimize flow, allows monitoring to detect and resolve clinical changes, facilitates weaning from ECMO, and contributes to the post-implantation assessment of possible complications.13–15Table 1 summarizes the echocardiographic parameters that should be evaluated and recorded in patients with ECMO.
Echocardiographic parameters to be evaluated in patients with ECMO.
| Structure | Parameter | Reference |
|---|---|---|
| Left ventricle | ||
| • Morphology | Myocardial size and thickness | |
| Septal position | ||
| Presence of autocontrast/thrombi | ||
| • Systolic function | Ejection fraction | |
| Segmental motility | ||
| Mitral S wave (TDI) | > 6 cm/s | |
| Velocity and size of VTI of LVOT | > 1 m/s and >10 cm | |
| • Diastolic function | E/A ratio of transmitral flow | <2 |
| EDT of mitral flow | >150 ms | |
| E/e’ ratio of the mitral ring | <8 | |
| Mitral and aortic valves | Diagnosis and grading of possible insufficiency and/or stenosis | |
| Left atrium | Size and volume | <35 ml/m2 |
| Pulmonary vein flow | S wave > D | |
| Right ventricle | ||
| • Morphology | RV/LV end-diastolic area ratio | <0.6 |
| Septal position and motion | ||
| Ventricular geometry | Triangular | |
| Eccentricity index | 1 | |
| Myocardial thickness | <8 mm | |
| • Systolic function | TAPSE | >16 mm |
| Tricuspid S’ wave (TDI) | >10 cm/s | |
| Shortening fraction | >35% | |
| McConnell sign | ||
| • Diastolic function | Tricuspid flow E/A ratio | <2 |
| Tricuspid and pulmonary valves | Tricuspid insufficiency for estimation of PAPs | <3 m/s |
| Pulmonary flow (Tac) | >120 ms | |
| Right atrium | Area | <20 cm2 |
| Structures (valves, Chiari, coronary sinus) | ||
| Septal integrity (foramen ovale) | ||
| RA-LA shunt (color Doppler ± shaken saline test) | ||
| Inferior/superior vena cava | Size and respiratory variation | <20 cm |
| Position of cannulas | ||
| Thrombi | ||
| Aorta | Thrombosis/atheromatosis/dissection | |
| Pericardium/pleura | Effusion (characteristics and grading) | |
TDI: tissue Doppler; VTI: velocity-time integral; LVOT: left ventricular outflow tract; EDT: E wave deceleration time; RV: right ventricle; LV: left ventricle; TAPSE: tricuspid annular plane systolic excursion; PAPs: systolic pulmonary artery pressure; Tac: acceleration time; RA-LA: right atrium–left atrium.
Despite its great usefulness, the use of ultrasound in patients with ECMO has important limitations. In the case of two-dimensional (2D) explorations, imaging acquisition may be adversely affected by both the patient’s position (dorsal or even prone decubitus) and the presence of invasive mechanical ventilation or devices such as vascular catheters, tubes or drains. Furthermore, the functional evaluation and interpretation of the recordings that will guide ECMO adjustment cannot be made by any operator, since advanced technical as well as clinical knowledge is needed. The level of knowledge influences decisions and adjustments that clearly may have an impact on the course of the patient.
Although there are different configurations,16 the present review focuses on the usefulness of ultrasound in patients with veno-venous (VV) and veno-arterial (VA) ECMO.
Veno-venous (VV) ECMOPre-VV ECMO evaluationPre-implant echographic evaluation of VV ECMO should be made on a routine basis, since different conditions need to be considered in order to select the type of assist and the optimum configuration.
The presence of severe left ventricular (LV) dysfunction refractory to inotropic drug treatment may require a change in configuration from VV to VA, or to veno-arterial-venous (VAV) mode.14 In addition to echocardiographic evaluation (left ventricular ejection fraction (LVEF) < 30% and/or left ventricular outflow tract (LVOT) velocity-time integral (VTI) < 10 cm), the presence of persistently elevated lactate concentrations (>5 mmol/l), central venous saturation <55%, cardiac index <2.1, the presence of arrhythmia with hemodynamic alterations, cardiac arrest and a vasoactive inotropic score >50 points during one hour or >45 points during 8 h, are factors that may prove useful for predicting claudication with VV ECMO.17
In evaluating VV ECMO implantation, another essential factor is right ventricular (RV) function. Many patients with severe acute respiratory distress syndrome (ARDS) present pulmonary hypertension and RV dysfunction in relation to hypoxia, hypercapnia, increased airway pressure and mechanical ventilation. Veno-venous ECMO, by correcting hypoxia and hypercapnia, reduces afterload and can improve RV function, thus correcting the hemodynamic instability. In the event of significant right ventricular dysfunction, echocardiographic monitoring is important, and if shock persists and the echocardiographic parameters fail to improve despite VV ECMO, we should consider changing the strategy to VA or VAV. The evaluation of RV dysfunction must consider the following: tricuspid annular plane systolic excursion (TAPSE) < 16 mm, S’ wave < 10 cm/s, shortening fraction (SF) < 35%, RV/LV end-diastolic area ratio > 0.6 (significant) and >1 (severe) or flattening/bulging (“D” form) of the interventricular septum in both systole and diastole (Supplementary material 1). This inter-dependence can be quantified using the ventricular eccentricity index, which is the ratio between the septum-inferior surface and anterior surface-inferior surface diameter in systole and diastole, with a normal value of 1.18
On the other hand, we should evaluate the existence of underlying disease conditions or anatomical alterations that may contraindicate implantation.14 In this regard, severe tricuspid valve disease (insufficiency and/or stenosis) may impair ECMO oxygenated blood flow from the right atrium (RA) to the LV. The presence of a persistent foramen ovale or interatrial communication, during weaning from ECMO, could increase the right-side pressures and generate a right-left shunt affecting oxygenation and even making removal of the device necessary. A prominent Chiari network may complicate the placement of the cannula and guide it toward the interatrial septum. The presence of a coronary sinus dilated by a left superior vena cava (SVC), if accidentally cannulated for the return, may drain the blood towards the left arm instead of to the RA.
Lastly, we must choose the best cannulation strategy according to the characteristics of the vascular accesses, emphasizing the presence of thrombi and/or anatomical variants/anomalies (Supplementary material 2).
Cannulation and start of VV supportThe systematic use of ultrasound is recommended during the different cannulation phases19:
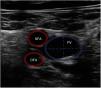
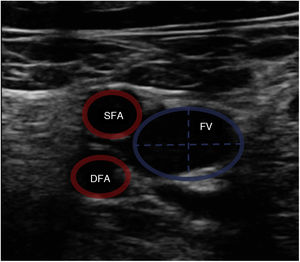
Vascular ultrasound allows us to measure vessel diameter to select the optimum cannula size (Fig. 1). The following formula is used for this purpose: cannula caliber (French (Fr)) = 3 × vessel diameter (mm). The largest cannula size should be used for both drainage and return, in order to ensure the greatest flow possible. Ultrasound-guided vascular puncture increases the safety and success rate at the first attempt, reducing the risk of local complications (arterial cannulation, cannulation of the saphenofemoral junction or transfixation of the inguinal ligament).20,11
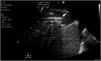
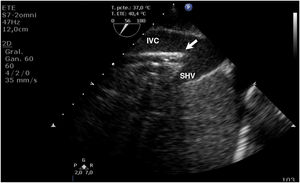
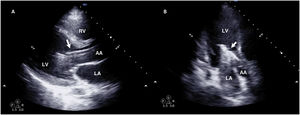
During cannulation, using ultrasound, we can check the intravascular insertion of the guides and subsequently of the cannulas. In the case of using a double-lumen cannula, transesophageal echocardiography (TEE) is essential, since serious complications may occur during implantation (perforation of the RA or cava superior, migration towards the RV), and we must check the correct orientation of the return flow towards the tricuspid valve.21 In cannulation with a simple double cannula, we can use transthoracic echocardiography (TTE) or TEE in the absence of a good acoustic window. When using the femoral-jugular configuration, the draining cannula must be located in the inferior cava below the left suprahepatic vein, and the tip of the return cannula must be positioned at RA level (Fig. 2) (Supplementary material 3). In the femoral-femoral configuration, the draining cannula should be positioned in the inferior cava and the return cannula in the RA. The distance between the two cannulas should be at least 10 cm to avoid recirculation.
Management of the patient with VV ECMODuring support, ultrasound helps us to assess the pulmonary response to the treatment, with dynamic monitoring of the hemodynamic changes and the detection of possible complications.3 For this purpose, we assess the following:
- -
Cannulas: daily monitoring is required of the correct positioning of the cannulas using echocardiography, assisted by the plain thorax-abdominal radiographic study. Mobilization of the cannulas can have an impact on oxygenation through an increase in recirculation, and moreover increases the risk of accidental decannulation. Changes in intra-circuit pressures can indicate possible migration of the cannulas, and ultrasound assessment can quickly confirm this complication. On the other hand, we should assess the appearance of thrombi around or within the cannulas (Supplementary material 4). This complication requires the optimization of anticoagulation, and a change in cannula and/or location may be needed.
- -
Right ventricle function may be impaired in the context of ARDS or secondary to concomitant complications such as pulmonary thromboembolism (PTE). The existence of echocardiographic evidence of severe ventricular dysfunction together with refractory hemodynamic instability may suggest the need for a change in configuration to VA or VVA.
- -
Left ventricle function should be evaluated using TTE/TEE. The presence of LVEF < 30%, VTI of the LVOT < 10 cm, E/A > 2 and/or a mitral flow E wave deceleration time (EDT) < 150 ms is suggestive of LV dysfunction, increased left-side pressures, and the possible need for a configuration change.
- -
Intracavitary or heart valve thrombi: The presence of such thrombi, in the same way as the presence of peri-cannula thrombi, requires the optimization of anticoagulation and should alert us to the risk of hemodynamic complications.
- -
Pulmonary ultrasound allows easy, dynamic and safe monitoring of pulmonary response to the treatment and the evolution of the primary disease condition based on the Lung Ultrasound Score (LUS). This score divides the lung areas into 12 regions (6 for each hemithorax) and assigns a score of 0–3 to each of them. The poorest score observed in each zone is recorded. We in turn speak of pattern A (0 points) in the presence of lung sliding with A-lines and ≤2 isolated B lines per intercostal space; pattern B1 (1 point) in the presence of ≥3 isolated non-coalescent B lines; pattern B2 (2 points) in the presence of coalescent B lines or “white lung” with or without small subpleural consolidations; and pattern C (3 points) in the presence of extensive lung consolidation (small subpleural consolidations are excluded).22,23
Ultrasound contributes to the diagnosis of complications during VV ECMO, and in some cases is of help in treating them.
In the case of refractory hypoxia, ultrasound helps us to evaluate the position of the cannulas as a cause of recirculation and also to assess cardiac output (CO) in order to examine the relationship between ECMO flow and CO of the patient, or QECMO/QCO ratio. A low ratio (≤60%) reflects insufficient ECMO flow for the existing CO; the first option, in this case, is therefore to increase the ECMO flow. If this is not possible, then in addition to controlling the underlying cause, we can adopt other measures such as the control of temperature or the use of beta blockers to reduce CO. In contrast, when the ECMO flow is adequate for the existing CO (>60%), in the presence of hypoxemia we should assume that there is a greater flow of deoxygenated blood that does not pass through the circuit, and should consider measures to optimize blood oxygenation, such as patient prone decubitus in ECMO.8,24
Ultrasound also allows the early and safe detection of pulmonary complications:
- -
Pneumothorax: the diagnosis of pneumothorax is established by the presence of two signs:
- •
Abolished lung sliding with/without the presence of E lines.
- •
Lung point, corresponding to the place of contact between the collapsed lung and the collection of air from the pneumothorax. This is a dynamic sign with a specificity of 100% that shows alternation between normal sliding (seashore sign) in inspiration and abolished sliding (stratosphere or barcode sign) during expiration in 2D and M mode (Supplementary material 5).
- •
- -
Pleural effusion: this is seen as a generally echo-free anechoic space above the diaphragm. The presence of a heterogeneous image or with enhanced echogenicity can suggest blood, and the appearance of septae in the effusion is indicative of organized pleural effusion.
- -
Bronchogram: this is observed as a hypoechogenic subpleural zone containing hyperechogenic images (air bronchogram) or a hypoechogenic content with hyperechogenic walls (fluid bronchogram). The presentation may be static (atelectasis) or dynamic (pneumonia) (Supplementary material 6).
- -
Hepatization/condensation: this is observed when the echographic density of the lung is similar to that of the liver.
In patients showing respiratory improvement and in which weaning of VV ECMO is considered, echocardiography can help us to evaluate the response of the RV and the signs of PHT on reducing assist. On the other hand, before removing the cannulas, echocardiography can be used to evaluate the existence of intraatrial thrombi that may delay weaning, increasing anticoagulation in order to try to reduce the size of the thrombi and prevent them from migrating during decannulation.
After withdrawing ECMO, it is necessary to assess the persistence of thrombi in the RA, cava and/or lower extremities (Supplementary materials 7 and 8). This is very useful in clinical follow-up for guiding the required duration of post-assist anticoagulation, diagnosing the appearance of deep vein thrombi in the vessels that have been cannulated, or identifying the appearance of PTE that may require other coadjuvant treatments (thrombectomy, cava filters).25
Veno-arterial (VA) ECMOPre-VA ECMO evaluationThere are different indications (cardiogenic shock in acute myocardial infarction, myocarditis or intoxications, postcardiotomy shock, PTE, cardiac arrest, etc.) for ECMO as circulatory assist, and the technique may involve different configurations (peripheral, central, hybrid).26 Therefore, and unless there is a contraindication or the situation does not allow the use of ultrasound, the latter is essential as an exploratory tool before implantation.
Basal TTE/TEE is required to evaluate both ventricular and atrial interdependence, function and size. We should seek possible anatomical defects (communications, thrombi, prominent valves, etc.) or aortic disorders (dissection, aneurysms) that may complicate or even impede the implantation of the device. In addition, we must detect the existence of valve anomalies (mitral or aortic valve insufficiency) that might worsen after starting the treatment (Supplementary material 9), or which may even require prior surgery.
Along with the cardiac evaluation, we should assess the possible presence of effusion (especially pericardial effusion) or vascular disease (arteriosclerosis, thrombosis) that may worsen or appear in relation to the implant. The presence of such disorders may require a configuration change (central or peripheral) or access (right/left or femoral/subclavian).14,15
In addition to the echographic signs, it is important to take into account and register the hemodynamic conditions, measures of support (mechanical ventilation, counterpulsation balloon, etc.) and drug treatments (type and dose of vasoactive medication, inotropic agents or vasodilators) that may influence interpretation.
Cannulation and start of VA supportEvaluation, vascular puncture and positioning of the guides are carried out in the same way as in VV ECMO, and the same protocol is followed. With regard to the placement of the venous cannula, a multiperforated cannula is indicated in VA support and should be positioned at the RA, with the tip at the SVC level, in order to secure the greatest drainage possible (Supplementary material 10).
In percutaneous femoral arterial cannulation, vascular ultrasound is very useful for the correct evaluation of the point of insertion, avoiding atheroma plaques or vascular alterations. When inserting the return cannula, we must puncture and insert the guides in the cranial direction, above the femoral bifurcation.27 Since the cannula can completely occlude the arterial lumen and therefore affect flow in the cannulated extremity, ultrasound-guided cannulation should be performed of the superficial femoral artery in the caudad direction to ensure perfusion of the extremity.7 Once the guide has been placed at the arterial level, TTE/TEE can identify its position at the descending aorta, confirming the correct arterial access of the guide. This is useful in cases of very low flow or pulsatility, as in cardiac arrest, where peripheral evaluation may prove difficult and urgent cannulation is required.28
On starting assist, TTE/TEE allows us to evaluate the situation, size and degree of decompression of the heart cavities; assess septal position as the expression of ventricular interdependence; and analyze volemia/preload that may affect ECMO flow and the development of early complications such as pericardial effusion, aortic valve closure or increased valve insufficiencies secondary to the increase in LV afterload conditioned by ECMO.29
Management of the patient with VA ECMOIt must be taken into account that most of the monitoring systems (transpulmonary thermodilution, pulse profile analysis) present artifacts due to the hemodynamic effect of ECMO; TTE/TEE therefore must be included in the daily evaluation of these patients. Based on this exploration we can assess and compare the following versus the basal exploration15:
- -
Biventricular contractility and size, which reflects the evolution of the cardiac condition for which ECMO was prescribed.
- -
The presence of echocardiographic signs (E/A ratio, E wave deceleration gradient, E/e’ ratio) of high left end-diastolic pressures indicating inadequate ventricular function.
- -
The appearance or progression of mitral valve insufficiency due to ventricular dilatation and/or increased pressures (Supplementary material 11).
- -
The presence of auto contrast suggesting blood stasis or even intracavitary thrombi that can give rise to distal embolism (Supplementary material 12).
- -
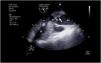
The increase in afterload conditioned by ECMO, together with low left ventricular contractility, can adversely affect aortic valve opening, with early closure of the valve (Supplementary material 13), or the valve may even remain closed (Fig. 3 and Supplementary material 14).
- -
Appearance or progression of aortic insufficiency, impeding LV emptying and perpetuating the high-end-diastolic pressures (Supplementary material 15).
- -
Stroke volume and native CO using VTI of the LVOT to assess the evolution of ventricular function. It must be taken into account that the volume circulating in the lung territory, and which reaches the left cavities can vary according to the presence of aortopulmonary collaterals, bronchial circulation and especially ECMO flow.
- -
The presence and characteristics of pericardial effusion. In these cases, it must be taken into account that the data referred to right cavity collapse can be increased by the negative pressure generated by ECMO, though not so in the left cavities; consequently, signs of tamponade must be correlated with the clinical situation and the behavior of ECMO.
- -
The position and flow of the cannulas. Patient mobilization can cause the cannulas to shift position. They consequently must be evaluated on a daily basis, with an assessment of the appearance of adhered thrombi that may embolize or even affect ECMO flow.
In addition to echocardiography, daily monitoring can involve pleuropulmonary ultrasound for evaluating both the lung parenchyma and the diaphragm or pleura.29,30 Transcranial Doppler poses limitations because it experiences artifacts due to the continuous flow of ECMO and other devices (Intra aortic balloon counterpulsation (IAoBC), Impella®) (Supplementary material 16); nevertheless, it also may be useful in the evaluation of cerebral hemodynamics and even in diagnosing brain death.31,32
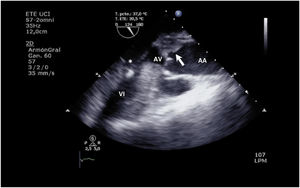
Lastly, many patients with VA ECMO may also carry some other left ventricular assist device (IAoBC, Impella®),33 and its position and function must be monitored as well.12 In the case of IAoBC, we can use TTE/TEE to observe insufflation of the balloon at the level of the descending aorta, with its distal extremity at the left subclavian root level (Supplementary material 17). Impella® can be explored with both TTE (long-axis parasternal or three-chamber apical) and TEE (mid-esophageal 120º), and we should see the device entering from the ascending aorta and the inlet or suction zone at 3–4 cm from the aortic valve34,35 (Fig. 4 and Supplementary material 18).
Resolution of VA ECMO problemsOne of the most frequent complications in patients with VA ECMO is a decrease in flow. Using TTE/TEE, we can check the positioning of the cannulas with suction events (Supplementary material 19), the presence of thrombi or the presence of pericardial effusion accounting for the impossibility of filling of the device as the cause of diminished flow (Supplementary material 20). It must be taken into account that if there is no impairment of flow, the presence of pericardial effusion does not need to imply intervention. Nevertheless, we must monitor its characteristics and evolution, for in some cases the signs of cardiac tamponade only manifest at the time of withdrawal.
Aortic closure is a complication that must be resolved, since persistent closure gives rise to intraventricular blood stasis and increased left-side pressures that in turn lead to left cavity dilatation and ultimately persistent lung edema. If this happens, TTE can evidence “smoke” or even intraventricular thrombi and signs of high left-side pressures (E/A ratio >2 and/or a mitral flow deceleration time (EDT) of <150 ms). In addition to its diagnostic/evolutive usefulness, TTE can be of help in guiding the treatment of this complication through assessment of the response to the use or increase in inotropic treatment (Supplementary material 21), or in guiding the implantation of devices (IAoBC/Impella®) or interventionism (atrial septostomy, apical drainage, left atrial VA ECMO (LAVA ECMO)) for alleviating left-side pressures.12
In patients with femoral-femoral VA ECMO, when ventricle function recovers and lung function has not yet improved, we can observe differential hypoxemia, north-south syndrome (or harlequin syndrome), due to upper trunk perfusion with poorly oxygenated blood from the pulmonary circulation and lower trunk perfusion with oxygenated blood from ECMO. This complication is usually detected by the determination of oxygenation (arterial blood gases, pulse oximetry) at the right upper extremity or cerebral level (near-infrared spectroscopy (NIRS)), requiring us to optimize native lung ventilation. In this respect, pulmonary ultrasound is useful for evaluating both the lung pattern and the possible response to the adopted measures.29
Weaning and post-decannulation usefulness of VA ECMOFor weaning from VA ECMO, the patient must be clinically (lactic acid <2 mmol/l, Pa/FiO2 > 200, improvement of organ dysfunction) and hemodynamically stable (mean blood pressure > 65 mmHg, pulse pressure > 20 mmHg, SvO2 > 65%), indicating that the underlying cause is controlled. Although there are no validated echocardiographic protocols or scientific recommendations as to when and how to perform weaning from ECMO, it is accepted that the patient must present echocardiographic signs of recovery of biventricular function (LVEF > 25%–30%, VTI of the LVOT > 10 cm, mitral ring S’ > 6 cm/s, TAPSE > 16 cm), with no evidence of high end-diastolic pressures that may worsen on suspending the assist measures.36 In the same way, as in VV ECMO, we must evaluate the presence of thrombi surrounding the cannula that could delay removal of the latter, or we at least should continue early with anticoagulation therapy once the cannula has been removed.
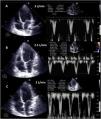
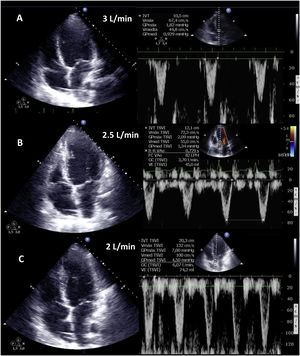
Weaning involves a progressive decrease (500 ml every 15–20 min) in VA ECMO support, gradually restoring cardiac preload. This requires adequate patient anticoagulation. We evaluate the hemodynamic (central venous pressure, mean and differential blood pressure, SvO2, SatO2) and echocardiographic repercussions of the procedure (LVEF, VTI, E wave, E’ wave or S’ of the lateral mitral ring) to a level (normally 1–1.5 l per min) indicating that the patient can tolerate suspension of the assist measures. During the latter, we check that LVEF is maintained or even exceeds 25%–30%, with VTI above 10 cm and an S’ wave of >6 cm/s (Fig. 5 and Supplementary material 22). In addition, we should also evaluate the behavior of the right-side cavities (TAPSE > 16 cm/S’ wave > 10 cm/s and RV size) and the possible appearance of signs of PHT (increase in peak velocity of tricuspid insufficiency (TI)) predicting RV failure.37–39
Echocardiographic evaluation of weaning in a patient with VA ECMO. Transthoracic echocardiography (TTE) and velocity-time integral (VTI) of the left ventricle outlet tract (LVOT) on reducing flow (A: 3 l/min, B: 2.5 l/min, C: 2 l/min) of VA ECMO. Note the increase in magnitude of VTI and consequently of stroke volume (SV) and cardiac output (CO).
After weaning, we perform the same controls as in the case of VV ECMO, taking special care in screening for intracavitary or intravascular thrombi that require the maintenance of anticoagulation therapy, or which may migrate.
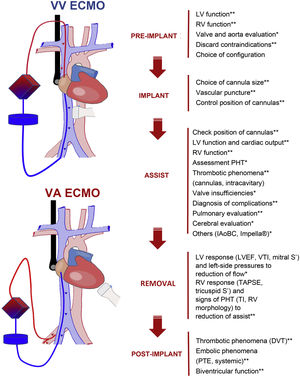
ConclusionsUltrasound in patients with ECMO is a key tool in all the assist phases. In preimplantation it is used to evaluate the vessels to be cannulated and biventricular function; during implantation, it is of help in ensuring safe and correct insertion of the cannulas; and in the assist phase, it is used to detect and treat complications and to study the pulmonary and/or cardiac evolution to ensure safe weaning (Fig. 6).
Taking into account its usefulness, ultrasound should be seen as a crucial instrument in the management of these patients, and the intensivists that perform the technique must have advanced knowledge of the anatomical, technical and functional aspects of the procedure. Further research is needed in this field, together with the development of scientific recommendations allowing correct training and improvement of patient outcomes.
Authors’ contributionsLMV, MPFG, RMB and HPC designed the study, conducted the literature review and wrote the manuscript. LMV, RMB and MPFG participated in the preparation of the tables and figures. LMV, MPFG, RMB and HPC reviewed the final version of the manuscript. All authors read and approved the final manuscript.
Conflict of interestsThe authors declare that they have no conflict of interests.