Pertussis, also known as “whooping cough,” is a highly contagious acute respiratory illness caused by the gram-negative intracellular coccobacillus Bordetella pertussis. Prior to the vaccine introduction, the pertussis was a devastating illness.1 Nowadays cyclical epidemics occur every two to five years and there has been a steady increase in reported cases with mortality occurring predominantly in young not vaccinated infants.1,2
B. pertussis is transmitted via aerosolized respiratory droplets. The organism adheres to ciliated respiratory epithelial cells of the upper respiratory tract and nasopharynx. It promotes cellular attachment, causes local and systemic tissue damage and interferes with host defence mechanisms.1 Histopathological analysis from cases of fatal pertussis revealed intracellular organisms in alveolar macrophages as well as the ciliated respiratory epithelial cells. This may explain the prolonged duration of cough. The most notable systemic laboratory manifestation is lymphocytosis, which is caused by pertussis toxin.2 As an intracellular organism, the usual host defence patterns activated in case of bacterial infection may associate a not typical response.1 The study by flow cytometry of this pattern through the expression of CD64, CD18, CD11b and CD11a in granulocytes could add useful data.3,4
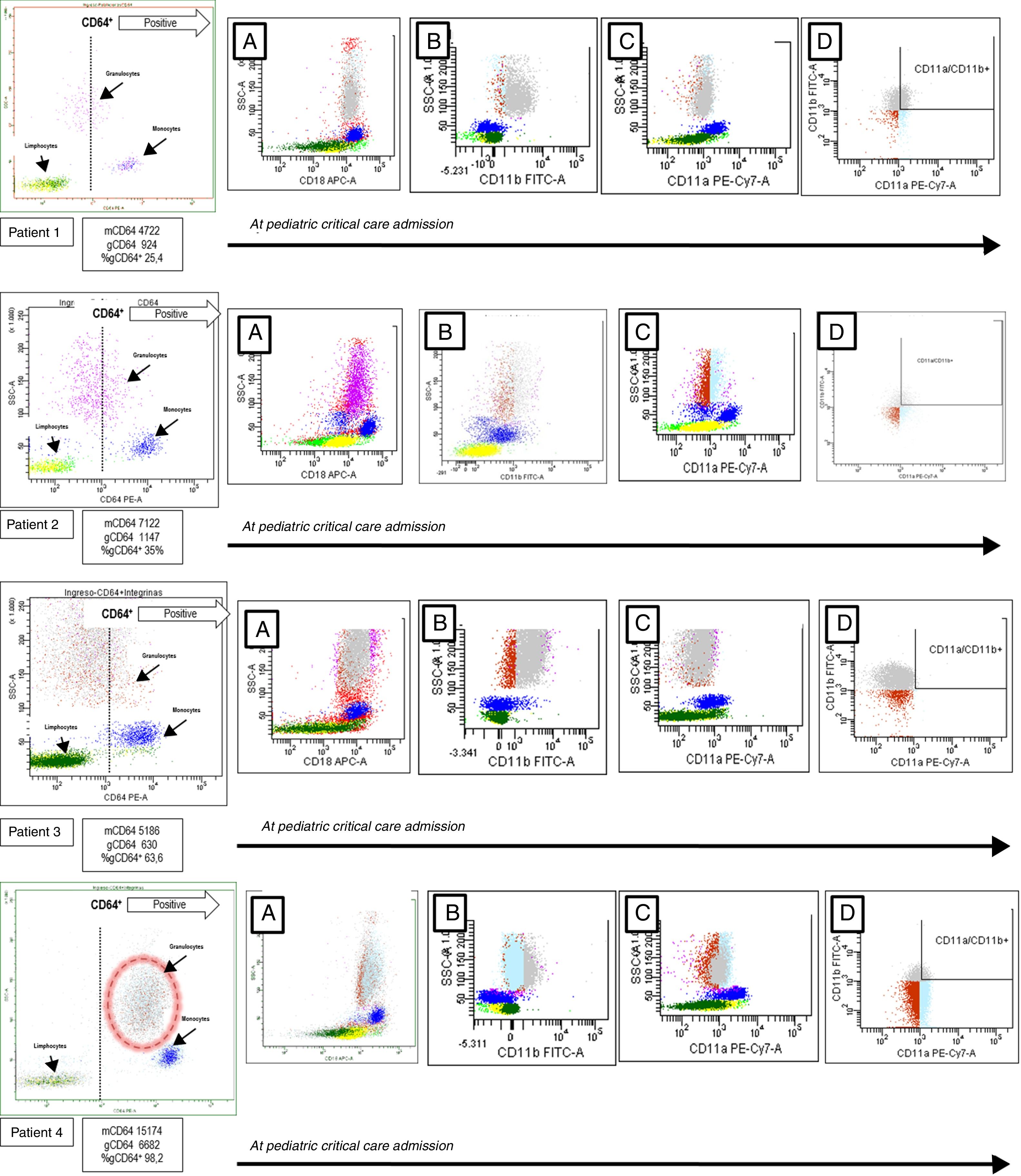
We study four children admitted to our pediatric critical care unit (PICU) from October 2015 to February 2016 because of B. pertussis infection (2 male, 2 female, mean age 48±8 days, mean±mean standard error). The patients were enrolled after obtaining a written informed consent. A blood sample was obtained at PICU admission to study the expression of CD64, CD18, CD11b and CD11a in granulocytes, using a BD FACS Canto II flow cytometer (Becton Dickinson, New York, USA). CD64 (clone 10.1), CD18 (clone CBR LFA-1/2), CD11b (clone CBRM1/5) and CD11a (clone HI111) monoclonal antibodies were from Biolegend®, San Diego. Cells viability was confirmed by 7-AAD staining. The flow cytometer settings and samples were prepared according to the manufacturer's instructions. Neutrophils, monocytes, and lymphocytes were identified on forward/side scatter dot-plot profiles and gated. The intensity of expression was measured as mean fluorescence intensity (MFI) and the positive cells were expressed as a percentage (Fig. 1). The patients were chronologically numbered. All of them were treated with azithromycin and received supportive treatment related with their needing. Respiratory support was the main problem in all of them. Blood cell counts and inflammatory biomarkers were obtained at the same time as flow cytometry (Table 1).
Flow cytometry analysis of patients with Bordetella pertussis infection. The granulocytes, monocytes, and lymphocytes were identified on dot-plot profile and gated by its CD64 expression. The positive CD64 region is marked by a discontinued line. In patient 4 the positive CD64 granulocytes are rounded by a discontinued circle. In each patient, and from left to right, the CD18 expression is showed in the A dot-plot, the CD11b expression is showed in the B dot-plot, CD11a expression is showed in the C dot-plot and granulocytes CD11b+/CD11a+ are showed in the D dot-plot. mCD64: mean fluorescence intensity or MFI of CD64 in monocytes; gCD64: MFI of CD64 in granulocytes; gCD64+: percentage of positive CD64 granulocytes.
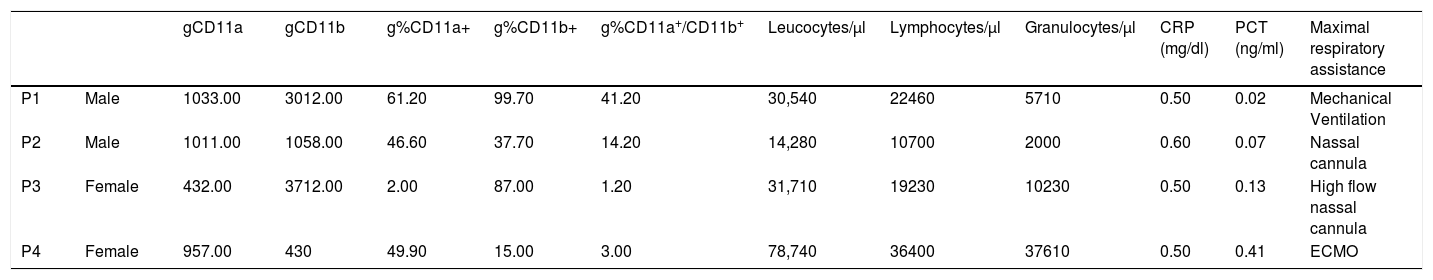
Blood count, acute phase reactants and granulocyte CD11a and CD11b expression at pediatric intensive care admission.
| gCD11a | gCD11b | g%CD11a+ | g%CD11b+ | g%CD11a+/CD11b+ | Leucocytes/μl | Lymphocytes/μl | Granulocytes/μl | CRP (mg/dl) | PCT (ng/ml) | Maximal respiratory assistance | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | Male | 1033.00 | 3012.00 | 61.20 | 99.70 | 41.20 | 30,540 | 22460 | 5710 | 0.50 | 0.02 | Mechanical Ventilation |
| P2 | Male | 1011.00 | 1058.00 | 46.60 | 37.70 | 14.20 | 14,280 | 10700 | 2000 | 0.60 | 0.07 | Nassal cannula |
| P3 | Female | 432.00 | 3712.00 | 2.00 | 87.00 | 1.20 | 31,710 | 19230 | 10230 | 0.50 | 0.13 | High flow nassal cannula |
| P4 | Female | 957.00 | 430 | 49.90 | 15.00 | 3.00 | 78,740 | 36400 | 37610 | 0.50 | 0.41 | ECMO |
P: patient; gCD11a: mean fluorescence intensity or MFI of CD11a in granulocytes; gCD11b: MFI of CD11b in granulocytes; g%CD11a+: percentage of granulocytes CD11a positive; g%CD11b+: percentage of granulocytes CD11b positive; g%CD11b+/CD11a+: percentage of granulocytes CD11a and CD11b positive; CRP: C reactive protein; PCT: procalcitonin; ECMO: extracorporeal membrane oxygenation.
Patient one to three showed good clinical evolution, with PICU discharge after two-three weeks of admission (mean days of PICU admission 16±6). Patient number four showed hemodynamic instability, coagulopathy and severe respiratory distress. Broad spectrum antibiotic was initiated and leukoreduction was done without improvement. Later, because of refractory hypoxemia despite aggressive approach, extracorporeal membrane oxygenation was initiated. The child died 48h after PICU admission and later the bronchoaspirate cultures done were positive to Klebsiella pneumonia.
As known, in case of B. pertussis infection, the use of common biomarkers is not useful to anticipate clinical complications and/or discard bacterial superinfection.1,2 As seen the C reactive protein (CRP) and procalcitonin (PCT) levels at admission were low in our patients, despite the confirmed bacterial infection and clinical status.1,2 The study by flow cytometry of host defence patterns could add new data in order to develop useful biomarkers.
B. pertussis, as an intracellular pathogen, associates a specific immune response that can be analyzed by flow cytometry. The CD64, an immunoglobulin G Fcγ receptor, is constitutively expressed in monocytes.5,6 Its expression levels increase in granulocytes in case of systemic inflammatory response (SIRS) and tissue damage. It has been described as a useful biomarker for diagnosis of bacterial infection, CD64 acts as a high-affinity receptor to bacteria.7,8 As observed, in cases one to three CD64 was expressed on monocytes but not in granulocytes. The percentages of CD64+ granulocytes (gCD64+) were always below 65% with a higher mean fluorescence intensity (MFI) of 1147. As expected the CD64 expression was not upregulated by this intracellular pathogen.8,9 This pattern was clearly different in patient 4 (see biomarkers in Table 1) which developed an increased CD64 MFI on monocytes and granulocytes with also a higher percentage of gCD64+. This infant showed a catastrophic evolution and a positive culture to K. pneumonia. The role of this pathogen in the granulocytes and the host defence developed was for sure critical.7
We also studied CD18, CD11b and CD11a (Fig. 1) to further complete the immunophenotypic analysis.4 The CD18, also known as integrin β2, participates in the adhesion and signaling of leukocytes. This molecule makes a complex with CD11b critical for the transendothelial migration, adhesion, phagocytosis, and neutrophil activation.4 The CD11a also associates with CD18 to form the lymphocyte function-associated antigen 1 or LFA-1. Expressed on all leukocytes it plays a central role in leukocyte cell–cell interactions and lymphocyte costimulation.10 In our serie, CD18 was expressed in all patients without differences. The granulocyte CD11a was similar in all children except in patient 3. The granulocyte CD11b expression by MFI was lower in patient 4 with also a low percentage of double positive granulocytes (CD11a+/CD11b+). These findings maybe are the expression of a differential immunological cellular pattern derived from the critical coinfection of this child.2,11
There are some limitations in this scientific letter. The work was conceived as a pilot study so we present a short number of cases with no controls to compare. Also, there is no information about medical history or previous diseases. We want to make clear that the main objective was to describe the expression of CD64, CD11a, CD11b, and CD18 in this disease.2,11 After our findings, more cases must be collected. The conclusions were obtained and deducted thanks to the previous knowledge about the molecules studied, so each patient act as self-control by the presence, absence, and expression of each molecule on cells.7 Related to the medical history, and based on the age of our patients, the molecules studied should not be influenced or modified in absence of a primary altered immune status (discarded by the studies done in the paper) or a previous or coexistent inflammatory disease.4,8 Finally, a not standardized flow cytometry protocol was applied. It must be validated and repeated prospectively in order to confirm its utility and viability.
In summary, as far as we know, we describe for the first time the CD64, CD18, CD11b and CD11a profile expression in children with B. pertussis infection.2 The expression of CD64 on granulocytes was not upregulated, which is consistent with an infection by an intracellular bacterium. Also among our cohort higher CD64 expression was related to bacterial superinfection and worst prognosis. CD18, CD11b and CD11a presence were similar in our patients except in the children who died, which showed in granulocytes a differential expression of CD11b and in CD11a+/CD11b percentage. To define its utility as a biomarker, and based on our findings, the study by flow cytometry of children with B. pertussis infection should be continued.
FundingThis work was funded by the Biomedicine Research Foundation of Hospital Infantil Universitario Niño Jesús.
Conflict of interestAll the authors do not have conflict of interest.
Work performed in Hospital Infantil Universitario Niño Jesús, Avenida Menéndez, Madrid, Spain.