These weeks of crisis are witnessing an unprecedented phenomenon in the healthcare setting: apart from the great volume of scientific articles which the specialized journals are quick to publish, we as professionals are continuously receiving different intervention protocols from national and international centers, video tutorials and expert opinions that have become an invaluable source of information provided we know how to use it adequately. Sharing medical information has its advantages if it is properly managed in the present epidemic. Medical decisions must be rational, profitable and respectful.
Rational, because such decisions must be supported by the best scientific evidence as defined in decreasing order by systemic reviews and meta-analyses, randomized clinical trials, cohort studies, registries, case-control series and case reports.
The appearance of this disease has led to the launching of many hastily designed clinical trials that do not always offer the desired methodological soundness, considering the circumstances, but which will yield results that are anxiously and urgently awaited. By administering certain treatments, we have not been fully capable of managing the scientific evidence out of fear of establishing suboptimal therapeutic strategies in seriously ill patients. It must be remembered that in Medicine, a number of experiences have shown that over time, less is actually more – thanks to initiatives such as “Slow medicine” in Italy, “Smarter medicine” in Switzerland, “Choosing wisely” of the ABIM Foundation in the United States and Canada, and “Do and not do” in Spain.1–3
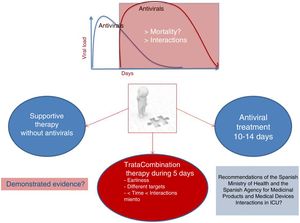
Current knowledge could be applied, caring for critical patients with SARS-CoV-2 infection without administering any antiviral therapy and providing respiratory and hemodynamic support, together with the adoption of other measures, according to the development of patient organ failure during admission to the Intensive Care Unit (ICU). Such measures and the prevention of infectious diseases related to healthcare must be priority concerns in the management of these patients, in which prolonged admission is expected. Everything else can be referred to as concomitant treatment measures of unconfirmed efficacy and with a risk of producing undesirable effects as a result of drug interactions and secondary complications. This premise may be extended to the use of corticosteroids and monoclonal antibodies or interleukin receptor antagonists.
Table 1 reports the reasons why antiviral drug treatments in severe SARS-CoV-2 infection in the critically ill are not justified.
Reasons why antiviral drug treatments in severe SARS-CoV-2 infection in the critically ill are not justified.
| Lack of rigorous studies demonstrating benefits |
|---|
| High probability of drug interactions with treatments commonly used in ICU |
| Extrapolation of clinical results in stable patients to critically ill patients |
| Assumption that antiviral action effective against other viruses is also effective against SARS-CoV-2 |
| In vitro activity does not necessarily imply in vivo activity |
| Erroneous assumption of equivalence between the different disease stages for starting treatment |
Profitable refers to the classical cost/benefit ratio. Cost is understood here in the broad sense of the word, referred more to a scarcity of resources in this crisis and to a more proportionate distribution of treatments than to a merely monetary concept. We are witnessing market competitivity that is generating inequalities among territories and also in patient selection practices. The still unanswered question is: “What patients stand to benefit most from antiviral therapies, and in what stages of the disease would they be most effective?”. Dealing with uncertainty while protecting those most vulnerable and those that may derive most benefit includes the selected and rationed distribution of these resources.
Respectful, because scientific bases require reliability and validation. The latter factor cannot be established in the current healthcare crisis due to the pandemic; as a result, the recommendations on antiviral treatments are referred to compassionate use. Hence, patients must be informed, and this must be duly reflected in the case history. In this pandemic, most informed consents are being obtained on an extraordinary basis by telephone, due to the visiting restriction policies of the hospitals. As soon as the patients recover their ability to decide, it is advisable to inform them of the decisions made, involve them in the process, and take their opinion into account – particularly in a situation of uncertainty regarding the potential benefits of the treatment.
Antiviral therapy in severe pneumonia due to SARS-CoV-2Different antiviral drugs have been postulated for the treatment of viral pneumonia due to SARS-CoV-2. The positioning of these drugs has been based on their activity against other infections produced by other different viruses, and on the clinical experience drawn from observational studies.
The clinical experiences with viruses such as influenza, Ebola, MERS, SARS and others, have led to the use of such antivirals in COVID-19 disease in both critical patients and in individuals not admitted to the ICU, with prescription rates of over 90%.4
The retrospective series comparing mortality-related factors have shown no differences associated to the use of antivirals.5
These drugs are being administered with the laudable aim of reducing the viral load, and it seems prudent to assume that the potential benefits would be obtained in the early stages of the disease, which is when viral replication is at its maximum. This implies that patients admitted to the ICU from the hospital ward after an average stay of one week are worsening more due to the body response to aggression than to the increase in viral load. This raises doubts as to the benefits of providing treatment in this stage. In these cases, managing scientific evidence instead of resorting to empiricism may be a prudent clinical strategy – particularly in view of the known interactions and side effects of these treatments, and not so much because of their hypothetical lack of activity against the virus. Fig. 1 shows different treatment strategies in the face of a lack of evidence.
Scientific evidenceA search of the website of the World Health Organization (WHO) regarding ongoing clinical trials shows that over 400 are currently registered – most of them in the recruitment phase or pending the start of recruitment. The most recent studies published in high impact factor journals report inconclusive results, generating uncertainty regarding treatment.6 An analysis critical of the possibilities of therapy, without taking into account the particularities of each individual patient may lead to error, since treatment response is variable, and many confounding factors can influence the prognosis of the critical patient, such as prior immune status, comorbidities, early intubation, mechanical ventilation mode, bacterial coinfection, healthcare-related infections, and concomitant treatments.
Mechanism of action of antiviral drugsThe physiopathological explanation of how SARS-CoV-2 uses its glycoprotein spicules to bind to the ACE receptors of the cell membrane7 and penetrate the cells to multiply and expand viral replication accounts for the different drugs that have been recommended for treatment. An advantage of these different treatment alternatives is that they are not mutually excluding, since they act on different targets, and their combined use is justified on the assumption that they may act synergically. Different antivirals have been used in the first recorded cases, though the published case series have not found one option to be superior to any other. A brief account is provided below of the drugs being used in Spain.
Chloroquine and hydroxychloroquine are not really antivirals, but are mentioned here because they have been among the most widely used drugs in Spain during the pandemic. Both are 4-aminoquinolines used for the prevention of malaria, and they act upon viral particle endocytosis and exocytosis. They consequently may inhibit penetration of the virus into the cells. The benefits of hydroxychloroquine are based on the fact that it is more effective, and the drug is moreover considered to have antiinflammatory properties; in this regard, severe respiratory distress is known to be associated to an increased inflammatory state. In vitro studies have showed the effects of hydroxychloroquine to be more potent.8,9
Another option is lopinavir/ritonavir. As protease inhibitors, these drugs prevent protein fragmentation. Studies in animal models and in vitro research have demonstrated their efficacy against viruses belonging to the same family, such as SARS and MERS.10 However, some investigators question their capacity to reduce the viral load in the lungs in the presence of an increased inflammatory response. This has caused the efficacy of such treatment to be questioned in critical patients subjected to mechanical ventilation.11 Administration is being made in combination with interferon: the MIRACLE trial has evidenced synergy when joint administration is carried out.12 The main weakness is the limited clinical experience available, and the fact that many interactions and side effects have been reported.
Unfortunately, the drug which in our opinion a priori appears most attractive for use in the critically ill – remdesivir – has been the least available option in Spanish ICUs. Remdesivir is a reverse transcriptase inhibitor that inhibits RNA-dependent RNA polymerase, and exhibits activity in both human coronavirus infection and in animal models. Different clinical trials are currently ongoing that will help clarify its efficacy against the virus in the more serious cases. The fact that the drug can be administered via the intravenous route implies that plasma levels can be reached without depending upon digestive tract absorption, which may prove erratic in formulations for administration through the nasogastric tube, as in critically ill patients subjected to mechanical ventilation.
Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun. 2020 Jan 10;11:222. http://dx.doi.org/10.1038/s41467-019-13940-6.13 The documented clinical experiences involving the use of remdesivir on a compassionate basis are promising, though it must be mentioned that in the study of Grein et al. there was no control group; patients receiving vasoactive drugs or with renal failure were excluded; and severity based on the SOFA or APACHE score was not reflected – thereby preventing us from knowing what kind of patients were treated. Furthermore, treatment was started 9–15 days after symptoms onset, and as we know, those patients who are able to pass this phase without respiratory failure usually evolve favorably.14Table 2 describes the potential advantages and limitations of remdesivir use in critical patients.
Advantages and limitations of remdesivir in the treatment of severe pneumonia due to SARS-CoV-2.
| Effective in vitro reduction of viral load |
|---|
| Intravenous administration in patients subjected to mechanical ventilation, avoiding administration through nasogastric tube |
| Adequate concentration in lung tissues |
| Few interactions |
| Restricted use in ICU during the SARS-CoV-2 pandemic |
| Lack of well designed studies in critical patients |
Most studies on the efficacy of antiviral drugs are referred to infections caused by viruses other than SARS-CoV-2, and involve treatment in early stages that differ from the stage at the time of critical patient admission to the ICU. Moreover, the studies on efficacy are more related to the reduction of viral load than to factors related to the real prognosis in terms of clinical course, mortality and improvement of organ failure.
The lack of selection of those patients that may benefit from the available treatments has caused the access to certain antivirals to be limited, and we must insist on the importance of the interactions which these drugs can have in the critically ill, where the foremost concern is the support of failing organs – fundamentally the lungs.
The possibility of acting upon different targets supports the recommendation to combine several treatments and thus seek to establish a synergic effect. In the event of treatment, it seems reasonable to start the latter in the early stages of the disease, which is when it makes sense to reduce the viral load. However, it does not seem justified to prolong the duration of treatment. On the other hand, it is strongly advisable to ensure close monitoring of the interactions and side effects, and to suspend treatment if such problems are detected, in view of the uncertainty of the potential benefits.
The current challenge is not only to determine which agents are effective against SARS-CoV-2, but also to define at which timepoint of the disease each proposed treatment measure may be most effective.
The formal limitations of the regulatory agencies in relation to pivotal clinical trials have classically implied that critical patients are excluded from such studies. It is therefore risky to extrapolate the data obtained from other types of patients. Correct and therefore ethical clinical practice only acquires social legitimation if it has been previously warranted by clinical research. Maximization of the potential clinical benefits must be sought while minimizing the possible negative effects of untested treatments.
FundingThis study has received no funding.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Estella A and Garnacho-Montero J. Del empirismo a la evidencia científica en el tratamiento con antivíricos en los casos graves de infección por Coronavirus en tiempos de epidemia. Med Intensiva. 2020;44:509–512.