Acute renal damage (ARD) is a frequent syndrome in hospitalized patients. It is well accepted that ARD susceptibility and outcome are related to environmental risk factors and to the patient premorbid status. Recently, host factors have also been recognized as important in ARD predisposition and evolution.
ObjectiveTo analyze genetic influences related to the risk and severity of ARD. Data source: MEDLINE search. Selection of studies: articles published in English or Spanish between 1/1/1995 and 31/5/2011, analyzing the association between genic polymorphisms and (a) ARD susceptibility in patients versus healthy controls or within groups of patients; or (b) ARD severity. Exclusion criteria: studies published only in abstract form, case reports or including patients less than 16 years of age, on chronic dialysis or having received a renal transplant. Data extraction: at least one investigator analyzed each manuscript and collected the information using a predefined form.
ResultsWe identified 12 relevant studies that included 4835 patients. Eleven genes showed polymorphisms related to ARD susceptibility or severity. They were related to cardiovascular regulation (ACE I/D, eNOS, FNMT and COMT), inflammatory response (TNFα, IL10, IL6, HIP-1α, EPO), oxidative stress (NAPH oxidase) and lipid metabolism (APO E). Only APO E, ACE and AT1 receptor have been analyzed in more than one study.
ConclusionARD susceptibility and severity are influenced by genetic factors, which are multiple and involve different physiopathological mechanisms.
El daño renal agudo (DRA) es un síndrome frecuente en el paciente hospitalizado. Los factores de riesgo asociados a su desarrollo y evolución clásicamente aceptados se encuentran en relación con el ambiente o la enfermedad de base del paciente. Sin embargo, en los últimos años se ha reconocido la influencia de los factores genéticos.
ObjetivoAnalizar la influencia de los polimorfismos genéticos en el riesgo de presentar y en la evolución del DRA. Fuente de datos: búsqueda electrónica en MEDLINE. Selección de estudios: manuscritos redactados en idioma inglés o español, publicados entre el 1/1/1995 y el 31/5/2011 y que analizaron la asociación entre polimorfismos genéticos y: (a) susceptibilidad a DRA entre pacientes versus controles sanos o entre diferentes grupos de pacientes; (b) gravedad del DRA. Criterios de exclusión: estudios publicados solo en forma de resumen, casos clínicos o estudios que incluyeran pacientes menores de 16 años, en diálisis crónica o con trasplante renal. Extracción de datos: al menos uno de los investigadores analizó cada artículo mediante formulario predefinido.
ResultadosSe encontraron 12 trabajos que incluyeron 4.835 pacientes. Once genes contienen polimorfismos asociados a la susceptibilidad o gravedad del DRA. Hemos clasificado estos genes de acuerdo con su función en aquellos que participan en la respuesta hemodinámica (ACE, eNOS, FNMT y COMT), respuesta inflamatoria (TNFα, IL10, IL6, HIP-1A, EPO), estrés oxidativo (NAPH oxidasa) y en el metabolismo lipídico (APOE). Solo los genes de APOE, ACE y receptor AT1 han sido analizados en más de un estudio.
ConclusiónLa susceptibilidad y gravedad del DRA están relacionadas con factores genéticos que están implicados en distintos mecanismos fisiopatológicos.
Acute renal injury (ARI) is a serious and frequent condition in critically ill patients. Its clinical expression varies greatly from elevations in serum creatinine concentration to anuria and the need for renal replacement therapy (RRT).1 The incidence of ARI among hospitalized patients is approximately 7%,2 but reaches 40% in critical patients with sepsis.3 In Spain, Herrera-Gutierrez et al.,4 in the context of a multicenter study conducted in 1999 (FRAMI), found the incidence of ARI upon admission to the Intensive Care Unit (ICU) to be 5.7%.
The host response to environmental stimuli is specific of each individual patient, and is conditioned by the underlying genetic profile (genotype). Two non-consanguineous individuals share 99.9% of their nucleotide sequence. However, the relatively small 0.1% differentiating portion suffices to determine individual character within one same species.5 The basis of this variability resides in genetic polymorphisms, which are variants in the DNA chain in a specific location (locus) that determine the existence of at least two different alleles.6 The most frequent allele is referred to as the native allele, while the rest are polymorphic alleles. These variants can comprise: (a) a single nucleotide, conforming a single nucleotide polymorphism (SNP); (b) a sequence, in which case the polymorphism is defined by the insertion or deletion of a fragment; or (c) variants in the number of repetitions of a given nucleotide sequence.6–9
Polymorphisms exert their influence: (a) directly, through the modification of risk mediated by the polymorphism; (b) as a “linked” group or haplotype, through the modification of risk mediated by a set of polymorphisms located in regions close to the DNA sequence, and which therefore do not combine; or (c) as a “non-linked” group or combination of polymorphisms, through the modification of risk mediated by genetic variants that are not inherited jointly and are not close to each other in the DNA chain, but which determine a concrete risk if they coincide within the genome of one same individual.10
The aim of the present systematic review is to analyze the existing evidence on the influence of genetic polymorphisms upon the risk of developing ARI and its outcome.
Material and methodsThe review included articles published in English or Spanish analyzing the association between polymorphisms and: (a) susceptibility to ARI in patients versus healthy individuals, and between different groups of patients; (b) the severity of ARI. We excluded studies published only in abstract form, clinical cases, or studies including patients under 16 years of age, on chronic dialysis, or subjected to renal transplantation.
Search strategyA search was conducted in the United States National Institute of Health, National Library of Medicine (MEDLINE) of those studies published between 1 January 1995 and 31 May 2011, using the following Medical Subject Heading (MeSH) terms: «Acute Kidney Injury/genetics»; «Acute Kidney Injury» and «Genes»; «Acute Kidney Injury» and «Polymorphism, Genetic»; «Acute Kidney Injury» and «Disease Susceptibility»; «Acute Kidney Injury» and «Genetic Predisposition to Disease»; «Acute Kidney Injury» and «Genetic Testing»; «Renal Replacement Therapy» and «Genes».
We selected all the titles and abstracts potentially meeting the inclusion criteria for manual evaluation of the full text. Posteriorly, a review was made of the literature citations included in the articles, with the purpose of identifying possible additional studies.
Given the particular characteristics of heart surgery (known timing of the injury, a surgical triggering factor in all cases, and postoperative recovery usually taking place in specialized units), we decided to structure the analysis around two population groups: (a) the general population; and (b) the postoperative period of heart surgery.
The following information was collected from each selected article: first author, date of publication, number of participants, origin of the population, study design, inclusion and exclusion criteria, polymorphism analyzed, character observed, and studied outcome estimations (odds ratio (OR), relative risk (RR)).
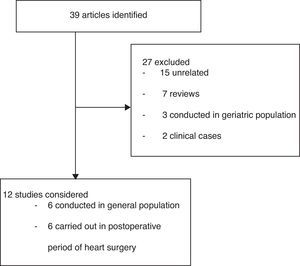
ResultsA total of 39 studies were identified; 27 were excluded and 12 were analyzed (Fig. 1). The polymorphisms associated to ARI susceptibility or outcome are presented in Table 1.
Genetic polymorphisms associated to acute renal injury.
| Author | Country | Observed phenotype | Polymorphism and/or haplotype | Risk allele | Result |
| General population | |||||
| Du Cheyron11 | France | ARI susceptibility among critical patients | ACE I/D | II | OR 6.5 (2.4–17.7) |
| Jaber13 | United Kingdom | Mortality associated to ARI | TNFα −308 | GA/AA | RR 2.47 (1.06–5.77) |
| Mortality associated to ARI | TNFα −308 & IL10 −1082 | GA/AA & AA | RR 5.17 (1.63–16.44) | ||
| Perianayagam14 | United States | Need for hemodialysis and/or mortality associated to ARI | NAPH Oxidase p22phox +242 | Allele T | OR 2.11 (1.10–3.75) |
| Kolyada15 | United States | Need for dialysis and/or mortality associated to ARI | HIF 1α +1772 | Allele T | OR 3.87 (1.70–8.01) |
| Need for mechanical ventilation.Hemodialysis or mortality associated to ARI | HIF 1α +1772 | Allele T | OR 4.73 (2–14-10.47) | ||
| Alam16 | United States | ARI susceptibility versus healthy controls | PNMT +1543 | Allele G | OR 2.19 (1.04–4.60) |
| Mortality associated to ARI | PNMT −161 | Allele A | OR 0.58 (0.35–0.99) | ||
| Shock associated to ARI | PNMT −161 | Allele A | OR 0.63 (0.40–1.00) | ||
| Mortality associated to ARI | PNMT −161/+1543 | A_/A_ | OR 0.60 (0.37–0.99) | ||
| Shock associated ARI | PNMT −161/+1543 | A_/A_ | OR 0.65 (0.42–1.00) | ||
| Postoperative period of heart surgery | |||||
| Popov17 | Germany | Need for renal replacement among patients with ARI | EPO rs 1617640 | AA | OR 3.25 (1.39–7.62) |
| Popov18 | Germany | Creatinine clearance | eNOS −786 | Allele T | Decreases. OR not indicated |
| Need for renal replacement during hospitalization | eNOS −786 | Allele T | Increases. OR not indicated | ||
| Mackensen19 | United States | Maximum plasma creatinine increase | APO E | ¿2 and/or ¿3 | Plasma creatinine increase |
| Haase-Fielitz20 | Australia | ARI susceptibility among postsurgery patients | COMT +472 | AA | OR 2.9 (1.2–7.0) |
| Isbir21 | Turkey | ARI susceptibility among postsurgery patients | ACE I/D | Allele D | Risk increase. OR not indicated |
| ARI susceptibility among postsurgery patients | APO E | ¿2 and/or ¿3 | Risk increase. OR not indicated | ||
| Stafford-Smith22 | United States | Maximum change in postoperative plasma creatinine | AGT894 & IL6 −572 | C & C | Increase in maximum creatinine variation versus baseline |
AGT: angiotensinogen; APO E: apolipoprotein E; COMT: catechol-O-methyltransferase; ACE: angiotensin-converting enzyme; eNOS: endothelial nitric oxide synthase; EPO: erythropoietin; HIF: hypoxia inducible factor; PNMT: phenylethanolamine N methyltransferase; IL6: interleukin 6; IL10: interleukin 10; NADPH oxidase: nicotinamide adenine dinucleotide phosphate oxidase; TNFα: tumor necrosis factor-alpha.
Du Cheyron et al.11 analyzed the influence of the insertion (I)/deletion (D) polymorphisms referred to angiotensin-converting enzyme (ACE) in a group of Caucasian patients over 18 years of age without chronic kidney disease, admitted to the ICU due to different disease conditions (cardiovascular, n=36; neurological, n=42; pulmonary, n=52; traumatic, n=8; gastrointestinal, n=20; and not specified, n=20). The multivariate analysis showed an age of over 75 years, the SAPS II score, the presence of infection, the need for vasopressors upon admission to the ICU, and genotype II («insertion» homozygote) to be independently associated to the development of ARI. With the purpose of evaluating the functionality of the genetic variant, the authors measured serum ACE activity recorded during the first 24h of admission to the ICU. They found that patients with genotype II and ID polymorphism had similar ACE levels (20±14U vs 22±18U; nonsignificant difference), though these levels in turn were lower than in DD patients (30±23U; p<0.05 vs the other groups). Recently, Pedroso et al.12 analyzed the association between the ACE I/D polymorphisms and −262 A/T with respect to the evolution of ARI as assessed by the renal SOFA score in a group of 153 patients over 18 years of age admitted to the ICU. The study excluded HIV-positive patients, pregnant or nursing women, and individuals receiving immunosuppressive treatment. No association was found between any of the genotypes and the evolution of ARI or the need for renal replacement therapy.
CytokinesJaber et al.13 analyzed the influence of SNP −308G/A referred to the tumor necrosis factor (TNF) α gene and SNP −1082G/A of the interleukin (IL) 10 gene upon the evolution of 67 hospitalized patients requiring dialysis as treatment for ARI. The percentages of survivors after 28 days of admission according to the combination of genotypes was as follows: 71% for TNFα GG and IL10 AA; 57% for TNFα GG and IL10 GG; 45% for TNFα AA or GA and IL10 GG; and 17% for TNFα GA or AA and IL10 AA. The influence of the genotypes upon cytokine secretion was evaluated ex vivo via the stimulation of leukocytes obtained from patients with Escherichia coli endotoxin. The authors found genotypes TNFα GA or AA to be associated to a greater concentration of TNFα versus GG (508pg/ml vs 161pg/ml; p=0.002). Similarly, the concentration was greater in patients with genotype IL10 GG versus IL10 AA (41pg/ml vs 77pg/ml; p=0.03).
Oxidative stressIn 200 individuals consecutively admitted to hospital due to ARI and in which the nephrologist was consulted, Perianayagam et al.14 analyzed the association between the polymorphisms of nicotinamide adenine dinucleotide phosphate oxidase (NADPH oxidase) p22phox +242C/T and catalase −262C/T and the clinical outcome. Patients on chronic dialysis, pregnant women, patients subjected to organ transplantation in the previous year, or subjects with obstructive renal disease were excluded from the study. The authors found allele T (TT or CT) in position +242 of the NADPHp22phox gene to be associated to longer hospital stay (17 days vs 11 days; p=0.02) and to an increased frequency of the combined outcome hemodialysis and/or mortality (adjusted for race, gender, age, SAPS II and chronic renal failure). In contrast, the catalase −262 polymorphism was not associated to any of the events analyzed. In plasma collected at the time of patient enrollment in the study, the nitrotyrosine concentrations were found to be greater among the carriers of the NADPH oxidase p22phox +242 TT genotype versus those with genotype CC.
Hypoxia-inducible factor 1α (HIF)Kolyada et al.15 analyzed the relationship between SNP HIF1α +1772C/T and the clinical outcome in 241 patients hospitalized due to ARI. The inclusion and exclusion criteria were the same as in the previous study by Perianayagam et al.14 Allele T (CT or TT) was associated to an increase in the combined outcomes: (a) risk of death and/or dialysis; and (b) death, dialysis and/or mechanical ventilation. The serum levels of vascular endothelial growth factor (VEGF)-A at the time of patient recruitment (54±7pg/ml vs 34±4pg/ml; p=0.008) were also greater in association to the mentioned allele.
Phenylethanolamine N methyltransferase (PNMT)Alam et al.16 studied the influence of polymorphisms PNMT −161 G/A and +1543A/G upon the susceptibility to ARI. They compared 194 patients hospitalized due to ARI and in whom the nephrologist had been consulted versus 767 non-hospitalized patients without kidney disorders. All were Caucasian adults. Patients on chronic dialysis, pregnant women, patients subjected to organ transplantation in the previous year, or subjects with evidence of obstructive renal disease were excluded from the study. Allele G of SNP PNMT +1543 was associated to an increased susceptibility to ARI, though without influencing the clinical outcome. In contrast, SNP −161A (AA or GA) was associated to an improved prognosis (lesser mortality). The carriers of haplotype −161A/+1543A showed a lesser risk of shock and death. Genotype PNMT −161 AA in turn was associated to a decrease in urine adrenalin concentration (AA 3.5ng/mg, GA 6.7ng/mg and GG 6.8ng/mg; p=0.04). The mean noradrenalin level tended to be greater in this same genotype (AA 29.1ng/mg; GA 16.7ng/mg and GG 11.8ng/mg; p=0.94).
Patients subjected to heart surgeryErythropoietin (EPO)Popov et al.17 analyzed the association between SNP rs1617640 referred to erythropoietin (EPO) and the susceptibility to ARI in 481 Caucasian patients subjected to heart surgery, without antecedents of renal replacement therapy. In the univariate analysis, the TT homozygous patients showed a greater need for renal replacement therapy.
Endothelial nitric oxide synthase (eNOS)Popov et al.,18 in a series of 497 Caucasian adults subjected to myocardial revascularization surgery, found allele C of SNP eNOS −786 to be associated to a decrease in postoperative creatinine clearance (55.8±31.3 vs 62.1±35.8; p=0.004) and to an increase in the need for renal replacement therapy. This study excluded individuals over 80 years of age and patients with known neoplastic disease, chronic dialysis or transplantation.
Apolipoprotein E (APO E)Mackensen et al.19 studied the influence of the APO E genotype and the severity of aortic atheromatosis upon the development of ARI following elective myocardial revascularization surgery in 130 patients. The study excluded individuals subjected to emergency surgery, with serious liver disease, stroke or chronic kidney disease. The renal function variables were similar among the different ¿4 (¿2 or ¿3) and ¿4 genotypes. However, the interaction between APO E and the severity of aortic atheromatosis influenced the peak serum creatinine levels during the postoperative period. In other words, for the same severity of aortic atheromatosis as assessed by esophageal Doppler ultrasound, the carriers of APO genotypes different from ¿4 showed higher serum creatinine concentrations.
Catechol-O-methyltransferase (COMT)Haase-Fielitz et al.20 studied the influence of SNP COMT +472 G/A in 260 Caucasian patients subjected to elective heart surgery. Individuals receiving COMT inhibitors or type A or B monoamine oxidase inhibitors (MAOIs), nitrites, sodium nitroprussiate via the intravenous route or high corticosteroid doses were excluded from the study, in the same way as patients with chronic renal dysfunction and patients subjected to emergency heart surgery. The authors found the AA homozygote genotype to be associated to a greater increase in serum creatinine concentration and to a greater need for renal replacement therapy in the postoperative period. The serum levels of adrenalin and noradrenalin 6h after the operation were higher among the carriers of the AA genotype, though there were no differences among the three groups in the preoperative period.
Multiple polymorphisms belonging to different pathwaysIsbir et al.21 found ACE allele D and the alleles different from ¿4 (¿2 or ¿3) in the APO E gene to be associated in the univariate analysis to an increased risk of ARI in 248 patients following elective myocardial revascularization surgery. However, SNP +1166 of the gene encoding for the angiotensin 1 (AT1) receptor did not influence the risk of ARI. The authors also found the carriers of ACE allele D to have significantly higher serum ACE concentrations at the time of anesthesia induction and 12h after surgery.
Stafford-Smith et al.22 published a sub-analysis of the Perioperative Genetics and Safety Outcome Study. They excluded patients on chronic dialysis, non-Caucasian or non-Afro-American ethnic groups, and 307 individuals lacking the required data. The study included 1464 Caucasians and 207 Afro-Americans subjected to elective myocardial revascularization surgery, with the analysis of 12 polymorphisms referred to the ACE, angiotensinogen (AGT), angiotensin (AT) 1 receptor, eNOS, IL6, TNFα and APO E genes. In the Caucasian population, and after adjusting the level of significance according to the multiple comparisons made, the authors found the combination of polymorphisms AGT +842C and IL6 −572C to be associated to a greater increase in the creatinine concentration difference versus the postoperative levels. In the Afro-American population, none of the differences reached statistical significance.
DiscussionThe present review has found that polymorphisms of 11 different genes are associated to acute renal injury (ARI) susceptibility or to the severity or prognosis of ARI in different populations, while four of the analyzed genes showed no such association. The review comprised 4835 patients, with a mean of 402 participants per study. Only two investigations16,22 involved over 900 patients. The reproducibility of the analyzed polymorphisms is scarce, being limited to the APO E gene,19,21,22 ACE I/D with its AT1 receptor,21,22 and ACE isolatedly.11,12 The results are difficult to interpret, since the inclusion criteria, the end events observed, the sample sizes and the statistical analyses differed from one study to another. As an example, two of the three studies that included APO E concluded that the alleles different from ¿4 are associated to a poorer outcome. The third article, involving an almost four times larger sample size, recorded no association. In a similar way, of the four studies that explored polymorphism ACE I/D, one found genotype II to be associated with increased risk,11 another found allele D to be associated with increased risk,21 and two studies reported no association.12,22
The main potential applications of genetic analysis in clinical practice are:
- a.
Risk identification and quantification. The application of genomic information to estimate the risk of developing ARI is currently not a part of routine practice despite the fact that the strength of the association (e.g., evaluated by means of the odds ratio (OR)) of the genetic and clinical factors is relatively similar. As an example, the OR range for the genetic factors varies between 0.58 and 6.50, depending on the polymorphism and event analyzed. On the other hand, in a recent study that included 1345 patients who were subjected to heart surgery,23 it was seen that the four clinical factors independently associated to ARI were: (i) anesthetic risk category 3 or 4 of the American Association of Anesthesiology (ASA score 3–4)(OR 2.60 [1.03–6.55]); (ii) forced expiratory volume in the first second (FEV1)(OR 0.55 [0.32–0.96]); (iii) the need for vasopressor drugs (OR 1.02 [1.0–1.03]); and (iv) the duration of anesthesia (OR 1.04 [1.00–1.08]).
- b.
Generation of new etiological and physiopathological knowledge. Identification of the polymorphisms associated to ARI and of the way in which they influence expression of the respective genes will increase our knowledge of the etiology and physiopathology of ARI.
- c.
Gene therapy. While still in the experimental phase, a number of procedures have already been evaluated, and designed to insert functional copies of genes that are defective or missing in the genome of a given individual.24 An example of this is transfection of the Bcl-2 gene in the rat kidney, with the aim of reducing ischemia-reperfusion damage through modulation of the apoptotic and necrotic pathways.25
- d.
Pharmacogenetics. The analysis of the variability of drug response according to the genotype of the individual can result in greater therapeutic benefits and a minimization of adverse effects.10 As an example, the last step in the formation of adrenalin from noradrenalin is mediated by the PNMT enzyme. Alam et al.16 found that allele A of SNP −161 PNMT is associated to lesser mortality, and that AA homozygotes present lower urine adrenalin levels and greater noradrenalin levels. It therefore could be postulated that carriers of polymorphism SNP −161AA of the PNMT gene would benefit from the use of non-catecholaminergic vasopressors instead of catecholaminergic agents. SNP COMT +472 G/A, which determines substitution of the amino acid valine by methionine in codon 158, reduces the concentration of the enzyme and favors its transformation into a thermolabile form.22,26 Since COMT is essential for catecholamine degradation in the kidney, the finding by Haase-Fielitz et al.20 that genotype AA is associated to an increased risk of ARI and shock could be related to the catecholamine desensitization phenomenon. This interpretation is supported by the fact that the serum concentrations of adrenalin and noradrenalin 6h after surgery were greater. The described genetic association and the suggested physiopathological hypothesis, if confirmed, would also be relevant, since patients with genotypes of this kind could represent another group capable of deriving benefit from the use of non-catecholaminergic vasopressors.
Despite the potential applications of this new genetic knowledge, the available information is still partial and difficult to interpret. As an illustrating example, oxidative stress is an important etiopathogenic factor in ARI. SNP p22phox +242C/T, which determines substitution of the amino acid histidine by tyrosine in position 72 of the polypeptide, modulates binding affinity to the heme group, and thus the activity of the enzyme.27–29 Allele T is associated to lesser respiratory activity in endothelial cells28 and in human neutrophils.29 However, the studies that have analyzed the influence of SNP +242 upon the lipid peroxidation markers have yielded conflicting results. Nitrotyrosine is the product of tyrosine nitration by reactive nitrogen species such as the peroxynitrite anion and nitrogen dioxide; as a result, it is regarded as a marker of oxidative stress dependent upon nitric oxide. Paradoxically, in the study published by Perianayagam et al.,14 the NADPH oxidase p22phox +242 TT genotype was associated to increased levels of nitrotyrosine. As a possible explanation for this, the investigators suggested the existence of linkage between this SNP and another still unknown polymorphism, which would be the true element responsible for exerting the genetic influence.
A second example of the difficulties found in the interpretation of the results is represented by SNP HIF 1α +1772. Hypoxia plays a role in the development of ARI. HIF 1α is activated in situations of oxygen deficiency and activates a series of genes that are responsible for increasing cell resistance to hypoxia.30 SNP HIF 1α +1772C/T, which determines substitution of the amino acid proline by serine in position 582 of the protein, is associated to an increment in the transcription of the gene. The finding by Kolyada et al.15 is unexpected, since a priori the increase in the expression of this group of genes should increase resistance to hypoxia. Possibly, increased expression of the gene generates an excessive response that could persist over time, even after resolution of the ischemia, and thus ultimately would turn into an adverse or maladaptive response.
Classical research focuses on identification of the components of biological systems.31 The tremendous pace of technological progress has allowed a shift in the paradigm toward a holistic approach in which complex biomolecular networks are considered globally, attempting to explain diseases or phenotypes on the basis of interactions among the host genome, genic expression and the environment—in consistency with a systems biology approach. The exploration of candidate genes was the strategy used in all the publications considered in our review. This methodology, of great strength when it comes to interpreting the results, involves analysis of the associations among polymorphisms of genes integrating the physiopathological pathways with observable characteristics. However, the studies are limited to relatively few polymorphisms. Studies that investigate the entire genome (genome-wide association studies, GWAS) allow global exploration of the variants included in the nucleotide sequence, and have been successful in identifying associations with many complex illnesses.32 This strategy has not yet been applied to ARI, however.
In recent years, the challenges found in genetic and genomic studies have increased. In ARI, knowledge would be required of genomic and exomic sequences, along with their functional characterization; of the order and magnitude of the activation/inhibition of certain genes; and of their interaction with the epigenetic regulation levels and with the environment.33–35
ConclusionsThe genetic structure of the patient influences susceptibility to ARI and the severity and outcome of the condition once established. Such new knowledge could be applied in clinical practice for: (a) risk identification and quantification; (b) the generation of new etiological and physiopathological knowledge; (c) the design of new treatments based on gene therapy; and (d) the prediction of individual patient response to certain drugs.
At present, all the published studies use an approach based on individual candidate genes, which substantially limits interpretation of the results. In future, studies capable of analyzing thousands of polymorphisms of the entire genome (GWAS) in a large number of people, of different ages, different ethnic groups and different geographical areas could help expand the existing knowledge.
Financial supportThe investigation that has produced these results received financial support from the Seventh Framework Program of the European Union PM7/2010, by virtue of funding agreement no. 26486, and the collaboration of the Instituto de Salud Carlos III (FIS 08/1726), the Fundación Mutua Madrileña (AP/67842009) and the Lilly Foundation-Spain.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Cardinal-Fernández P, et al. Determinantes genéticos del riesgo y pronóstico del daño renal agudo: una revisión sistemática. Med Intensiva. 2012;36:626–33.
Dr. Pablo Cardinal-Fernández is a grant holder of the Marie Curie Program (PI-NET: ITTN 264864).