The main study objectives were to describe the practice of mechanical ventilation over an 18-year period in Mexico, and estimate changes in mortality among critical patients subjected to invasive mechanical ventilation (IMV).
DesignA retrospective subanalysis of a prospective observational study conducted in 1998, 2004, 2010 and 2016 was carried out.
SettingIntensive Care Units (ICUs) in Mexico.
ParticipantsAdult patients consecutively enrolled in the ICU during one month and who underwent IMV for more than 12h or noninvasive mechanical ventilation for more than one hour. Follow-up was performed up to a maximum of 28 days after inclusion.
InterventionsNone.
Principal variables of interestAge, sex, severity upon admission as estimated by SAPS II, parameters of daily arterial blood gases, treatment and complication variables, date and status at discharge from the ICU and from hospital.
ResultsA total of 959 patients were included in 81 ICUs. Tidal volume (vt) decreased significantly both in patients with acute respiratory distress syndrome (ARDS) criteria (estimated 8.5mL/kg b.w. in 1998 to 6mL/kg in 2016; P<.001) and in patients without ARDS (estimated 9mL/kg b.w. in 1998 to 6mL/kg in 2016; P<.001). The ventilatory protective strategy (defined as vt <6mL/kg or <8mL/kg and a plateau pressure <30cmH2O) was: 19% in 1998, 44% in 2004, 58% in 2010 and 75% in 2016 (P<.001). The adjusted mortality rate in ICU over the 4 periods was: in 2004, odds ratio (OR) 1.05 (95% confidence interval, 95%CI: 0.73–1.72; P=.764); in 2010, OR 1.68 (95%CI: 1.13–2.48; P=.009); in 2016, OR 0.85 (95%CI: 0.60–1.20; P=.368).
ConclusionsThe clinical practice of IMV in Mexican ICUs has been modified over a period of 18 years. The most significant change is the ventilatory strategy based on low vt. These changes have not been associated with significant changes in mortality.
Los objetivos principales son describir la práctica de la ventilación mecánica en un periodo de 18 años en México y estimar los cambios en la mortalidad de los pacientes críticos con ventilación mecánica invasiva (VMI).
DiseñoSubanálisis retrospectivo de un estudio prospectivo y observacional en 1998, 2004, 2010 y 2016.
ÁmbitoUnidades de Cuidados Intensivos (UCI) de México.
ParticipantesPacientes adultos que ingresaron consecutivamente en la UCI, durante un mes y que recibieron VMI durante más de 12h o ventilación mecánica no invasiva durante más de una hora. El seguimiento se realizó hasta 28 días después de la inclusión.
IntervencionesNinguna.
Variables de interésEdad, sexo, gravedad al ingreso estimada por el SAPS II, parámetros de la gasometría arterial diaria, variables de tratamiento y complicaciones, fecha y estado al alta de la UCI y del hospital.
ResultadosSe incluyó a 959 pacientes en 81 UCI. El volumen corriente (VC) ha disminuido significativamente tanto en pacientes con criterios de SDRA (de 8,5ml/kg de peso estimado en 1998 a 6ml/kg en 2016; p < 0,001) como en enfermos sin SDRA (de 9ml/kg de peso estimado en 1998 a 6ml/kg en 2016; p < 0,001). La estrategia ventilatoria protectora (definida como VC < 6ml/kg o < 8ml/kg y una presión meseta <30 cmH2O) fue: 19% en 1998, 44% en 2004, 58% en 2010 y 75% en 2016 (p < 0,001). La mortalidad ajustada en UCI a lo largo de los 4 periodos fue: en 2004, oportunidad relativa (OR) 1,05 (IC 95%: 0,73-1,72; p=0,764); en 2010, OR 1,68 (IC 95%: 1,13-2,48; p=0,009); en 2016, OR 0,85 (IC 95%: 0,60-1,20; p=0,368).
ConclusionesLa práctica clínica de la VMI en las UCI de México se ha modificado a lo largo de un periodo de 18 años. El cambio más significativo es la estrategia ventilatoria basada en VC bajos. Estos cambios no se han asociado a cambios significativos en la mortalidad.
Over the last few decades numerous clinical trials have been conducted with patients admitted to Intensive Care Units (ICU) to prevent or reduce ventilator-associated lung injury—use of non-invasive ventilation1 (NIV), lung-protective ventilation,2,3 adjustment of positive end-expiratory pressure (PEEP),4 decubitus prone position,5 use of neuromuscular blockers6. Also, to reduce the duration of mechanical ventilation—proper use of sedatives7,8 and early identification of when to start weaning from mechanical ventilation.9 Some of these interventions, which initially for the management of patients with acute respiratory distress syndrome (ARDS) now seem to be applicable to all the patients on mechanical ventilation.10 The possible clinical impact of the results from these studies in the specific context of a country has not been described extensively.
The objectives of our analysis were these: (1) to describe the evolution of the clinical practice of mechanical ventilation in Mexico, (2) to estimate whether the changes seen have followed the current scientific evidence, and (3) to estimate whether mortality has changed over time.
Material and methodsThis is a retrospective subanalysis study of patients admitted to Mexican intensive care units (ICU) who participated in 4 prospective, observational, non-interventional, international studies on mechanical ventilation conducted in 1998,11 2004,12 2010,13 and 2016. All of them were prospective and observational studies of patients admitted for one month who received invasive mechanical ventilation for over than 12h or NIV for over an hour. The follow-up of the patients included was during the time of mechanical ventilation up to a maximum of 28 days after inclusion.
The following variables were recorded in all patients: age, sex, severity on admittance estimated by SAPS II, daily arterial blood gas test, treatment-associated variables (ventilatory parameters, sedation, neuromuscular blockers), and complications (ARDS, sepsis, ventilator-associated pneumonia, cardiovascular failure, kidney failure, liver failure, hematologic failure), date, and condition upon ICU and hospital discharge.
The protocol and the definitions used have previously been published.13 Only the researcher from each ICU knew the characteristics of the study in order to not influence the daily routine clinical practices (list of researchers in Annex). The study was approved by the ethics committee of each of the participating institutions and the need for informed consent was applied according to local regulations. The study was conducted in full compliance with the Strengthening the Reporting of Observational Studies in Epidemiology standards for cohort observational studies.14
The objective of this analysis is to evaluate whether the changes observed from the first study conducted back in 1998 until the last study was conducted in 2016 meet the following hypotheses resulting from the clinical trials published over the last few decades: (a) increased use of NIV, both for the management of patients with chronic lung disease and for the management of acute hypoxemic respiratory failure; (b) increased use of a protective ventilation strategy defined as low or adjusted tidal volumes to maintain a low plateau pressure and use of high PEEP in patients with ARDS; (c) evaluate whether protective ventilation is also used in patients without ARDS criteria; (d) evaluate changes in the weaning from mechanical ventilation such as the frequency of use of support pressure vs. spontaneous breathing trial using a T tube, both during the first weaning attempt and in the weaning of patients with difficult-prolonged weaning, and (e) describe the practice of tracheotomy at the ICU setting over time. Finally, we evaluate whether some of these changes have had an impact on mortality rate.
Statistical analysisContinuous variables are expressed as mean (standard deviation) or median (P25, P75) and qualitative variables are expressed as the absolute and relative frequency of each value of the variables. Fisher's bilateral exact test was used for dichotomic variables when the expected value of a cell was <5. The variables were compared using ANOVA univariate analyses or the chi-square test, as appropriate. P values <.05 were considered statistically significant.
To estimate the changes in mortality 28 days after starting mechanical ventilation we conducted a multivariate logistic analysis adjusted by baseline variables, ventilation parameters, and complications occurred during mechanical ventilation including the variables with significant P levels <.05. The risk of hospital mortality was expressed as odds ratio (OR) and a 95% confidence interval (95%CI).
For data analysis the Stata/SE 14.0 statistical software package for Windows was used (Stata Corporation College Station, Texas, United States).
ResultsGeneral characteristics and outcomesNine hundred and fifty-nine patients were included (408 patients in 1998, 119 patients in 2004, 132 patients in 2010, and 300 patients in 2016) admitted to 81 ICUs (37, 5, 15, and 42 Mexican ICUs, respectively, back in 1998, 2004, in 2010 and in 2016, respectively). None of these ICUs participated in the 4 studies. Both the characteristics of inclusion and the outcomes are shown in Table 1.
Baseline characteristics and outcomes of patients included in the 4 studies.
| 1998 (N=408) | 2004 (N=119) | 2010 (N=132) | 2016 (N=300) | P | |
|---|---|---|---|---|---|
| Age in years, mean (SD) | 53 (16) | 54 (19) | 51 (21) | 48 (20.5) | .001 |
| Sex, male, n (%) | 242 (60) | 79 (66) | 79 (59) | 198 (66) | .029 |
| SAPS II, points, mean (SD) | 53 (17) | 43 (16) | 36 (17) | 47 (18) | <.001 |
| Reason for starting mechanical ventilation, n (%) | |||||
| COPD | 24 (6) | 9 (8) | 10 (8) | 3 (1) | .002 |
| Asthma | 4 (1) | – | – | 1 (0.3) | .365 |
| CLD not COPD | – | 3 (2.5) | 1 (1) | 2 (1) | .023 |
| ARDS | 12 (3) | 8 (7) | 10 (8) | 11 (4) | .630 |
| Postoperative | 192 (47) | 16 (13) | 29 (22) | 47 (16) | <.001 |
| Acute pulmonary edema | 38 (9) | 3 (2.5) | 8 (6) | 14 (5) | .018 |
| Aspiration | 15 (4) | 2 (2) | 1 (1) | 4 (1) | .098 |
| Pneumonia | 30 (7) | 9 (8) | 11 (8) | 30 (10) | .063 |
| Sepsis | 14 (3) | 17 (14) | 13 (10) | 38 (13) | <.001 |
| Trauma | 74 (18) | 5 (4) | 6 (4.5) | 21 (7) | <.001 |
| Cardiac arrest | – | 3 (2.5) | 2 (1.7) | 9 (3.2) | .010 |
| Neurocritical disease | 19 (5) | 34 (29) | 30 (23) | 100 (33) | <.001 |
| Neuromuscular disease | 4 (1) | 3 (2.5) | – | 6 (2) | .020 |
| Other causes | 46 (11) | 7 (6) | 11 (8) | 14 (5) | .012 |
| Outcome | |||||
| Duration of mechanical ventilation, days, median (P25, P75) | 3 (2. 4) | 6 (4. 9) | 6 (4. 9) | 5 (3. 8) | <.001 |
| Days of ICU stay, median (P25, P75) | 4 (3. 6) | 7 (4. 11) | 8 (5. 14) | 7 (4. 11.5) | <.001 |
| Days of hospital stay, median (P25, P75) | 12 (8. 20) | 13 (7. 19) | 17 (10. 30) | 17 (8. 30) | <.001 |
| ICU mortality, n (%) | 62 (15) | 31 (26) | 26 (20) | 65 (22) | .027 |
| Mortality at the 28-day follow-up, n (%) | 67 (16) | 34 (29) | 30 (23) | 67 (22) | .019 |
| Hospital mortality, n (%) | 68 (18) | 38 (32.5) | 32 (30.5) | 85 (36) | <.001 |
ARDS, acute respiratory distress syndrome; CLD, chronic lung disease; COPD, chronic obstructive pulmonary disease; SAPS II, Simplified Acute Physiology Score.
The main changes in the characteristics of the patients since the first study have been that slightly younger patients have been included, which is perhaps the reason why the estimation of severity, calculated using the SAPS II, has decreased a little bit. However, the most relevant fact is the reason for starting mechanical ventilation. This, while in 1998 the main causes were patients with postoperative acute respiratory failure and trauma, as the disease progresses it is possible to see how the most common disease is infectious disease (pneumonia and sepsis) and, above all, neurological disease, that back in 2016 represented one third of the patients included.
Changes in clinical practiceNon-invasive ventilationTable 2 shows the evolution of the use of NIV. We can see a low incidence in the use of NIV without major changes through time. Except for the first study, failure in its application was very high, with high mortality rates among patients who require intubation after a period of NIV.
Evolution in the use of non-invasive ventilation.
| 1998N=34 | 2004N=3 | 2010N=12 | 2016N=15 | P | |
|---|---|---|---|---|---|
| Percentage of use of NIV | 8 | 2 | 9 | 5 | .045 |
| Age, years, mean (SD) | 57 (19) | 75 (4) | 67 (17) | 59 (21) | <.001 |
| SAPS II, points, mean (SD) | 51 (22) | 43 (3) | 55 (14) | 40 (18) | <.001 |
| Reason for starting NIV | |||||
| Chronic lung diseasea | 9/28(32) | 2/12(17) | 5/11(45) | 1/6 (17) | <.001 |
| Acute respiratory failure | 22/357 (6) | 1/70 (1) | 7/91 (8) | 14/188 (7) | <.001 |
| NIV failure | 6 (18) | 3 (100) | 5 (42) | 9 (60) | .002 |
| Overall mortality | 5 (15) | 0 | 2 (17) | 4 (27) | .739 |
| Mortality failure | 2/6 (33) | 0/3(0) | 2/5 (40) | 4/9 | .723 |
| Mortality success | 3/28 (11) | – | 0/7 (0) | 0/6 (0) | 1.000 |
NIV, non-invasive ventilation; SAPS II, Simplified Acute Physiology Score; SD, standard deviation.
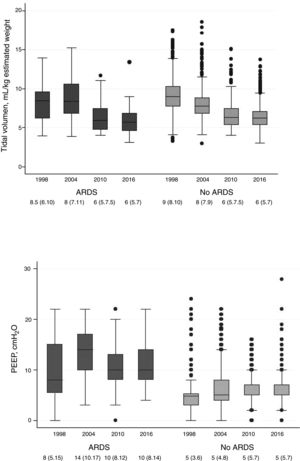
Fig. 1 shows the evolutions of tidal volume and PEEP in patients with ARDS criteria and without ARDS criteria.
Evolution of tidal volume (upper chart) and positive end-expiratory pressure (PEEP) (lower chart) in patients with acute respiratory distress syndrome (ARDS) criteria and without ARDS criteria over the 4 periods studied. The box plot shows the median (central line of the box) with the 25th and 75th percentiles (lower and upper edges of the box), ranges, and atypical values (dots) of the variables during all the days on mechanical ventilation.
In general, there is a tendency to ventilate with lower tidal volumes patients with ARDS criteria (from 8.5mL/kg of estimated weight in 1998 to 6mL/kg back in 2016; P<.001) and patients without ARDS criteria (from 9mL/kg in 1998 to 6mL/kg back in 2016; P<.001). Regarding programmed PEEP, less clinically relevant changes were seen (in patients with ARDS it increased from a median of 8cmH2O back in 1998, to 10cmH2O in 2016. In patients without ARDS the median remains almost the same).
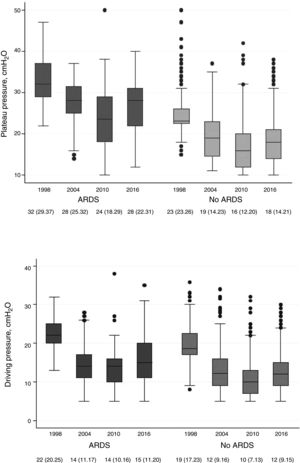
Fig. 2 shows the changes seen in the plateau pressure and in the driving pressure as a result of the adjustments made in the tidal volumes and PEEP. Also, these changes have induced differences in the percentage of days on mechanical ventilation when a protective ventilatory strategy was established (defined as tidal volume <6mL/kg or tidal volume <8mL/kg and plateau pressure or peak pressure <30cmH2O): 19% in 1998, 44% in 2004, 58% in 2010, and 75% back in 2016 (P<.001).
Evolution of the plateau pressure (upper chart) and the driving pressure in patients with acute respiratory distress syndrome (ARDS) criteria and without ARDS criteria over the 4 study periods. The box plot shows the median (central line of the box) with the 25th and 75th percentiles (lower and upper edges of the box), ranges, and atypical values (dots) of the variables during all the days on mechanical ventilation.
Table 3 summarizes the characteristics of those patients who were weaned from mechanical ventilation and extubated in a programmed way, the weaning methods, and the outcomes linked to weaning.
Evolution of weaning from mechanical ventilation.
| 1998N=255 | 2004N=74 | 2010N=60 | 2016N=193 | P | |
|---|---|---|---|---|---|
| Percentage of patients who are weaned from mechanical ventilation | 62 | 62 | 45 | 64 | .002 |
| Age, years, mean (SD) | 52 (15) | 53 (19) | 49 (20) | 48 (21) | <.001 |
| SAPS II, points, mean (SD) | 50 (17) | 40 (15) | 33 (16) | 44 (18) | <.001 |
| Days on mechanical ventilation before weaning, median (P25, P75) | 3 (2.4) | 6 (3.9) | 5 (3.7) | 4 (3.7) | <.001 |
| Method for the first weaning attempt % | <.001 | ||||
| Spontaneous breathing trial in % | 58 | 54 | 85 | 30 | |
| Gradual reduction of breathing support in % | 42 | 46 | 15 | 70 | |
| First attempt failure, n (%) | 48 (19) | 51 (69) | 10 (17) | 47 (24) | <.001 |
| Weaning method in patients with failed first attempt in % | <.001 | ||||
| Spontaneous breathing trial | 31 | 16 | 70 | 30 | |
| Gradual reduction of breathing support | 69 | 84 | 30 | 70 | |
| Days of weaning in patients with difficult–prolonged weaning | 4 (2.7) | 6 (5.9) | 7(6.10) | 6 (4.8) | <.001 |
| Ventilation time dedicated to weaning in % | 45 | 35 | 32 | 33 | <.001 |
| Reintubation within the first 48 h after extubation, n (%) | 12 (5) | 5 (7) | 7 (12) | 16 (8) | .177 |
SAPS II, Simplified Acute Physiology Score; SD, standard deviation.
A great variation is seen over time in the way mechanical weaning started, with a significant increase in the gradual reduction of ventilation support, basically support pressure, in the last cohort. A great variation is also seen in the percentage of patients who can be extubated at the first attempt. Except for the study from 2010, in the remaining studies the gradual reduction of ventilation support is accepted for patients with difficult-prolonged weaning. Within this group, although support pressure is predominant (from 21% in 1998 to 42% in 2016), synchronized intermittent mandatory ventilation (SIMV) plays a significant role with or without support pressure. It was used in 38% of the patients in 1998 and in 26% of the patients in 2016.
The percentage of tracheotomies performed over time has been 4% in 1998, 12% in 2004, 18% in 2010, and 13% back in 2016 (P<.001). The time elapsed until the tracheotomy was performed was a median of 11 days (P25: 5, P75: 20) in 1998; 11.5 days (10.17) in 2004, 15 days (8.18) in 2010, and 9 days (5.12) back in 2016 (P<.001). Excluding the study from 1998 that did not record the tracheotomy method, in the remaining ones most tracheostomies were surgical tracheostomies: 71% in 2004, 83% in 2010, and 79% in 2016 (P=.652).
Mortality changesTable 4 shows the evolution of gross and adjusted mortality on 28 days after the start of mechanical ventilation. Except for the increase of gross mortality seen in the 2004 cohort, in the remaining studies a slight decrease was seen until 2016. After adjusting the variables associated with mortality, no significant changes were seen in the evolution of mortality from 2004 to 2016.
Evolution of mortality risk on the 28th day.
| % | Gross mortality | P | Adjusted mortalitya | P | |
|---|---|---|---|---|---|
| Odds ratio, 95%CI | Odds ratio, 95%CI | ||||
| 1998 | 16 | 1 (reference) | 1 (reference) | ||
| 2004 | 29 | 2.03 (1.26 to 3.27) | .003 | 1.05 (0.73 to 1.52) | .764 |
| 2010 | 23 | 1.49 (0.92 to 2.43) | .102 | 1.68 (1.13 to 2.48) | .009 |
| 2016 | 22 | 1.46 (1.00 to 2.13) | .048 | 0.85 (0.60 to 1.20) | .368 |
Adjustment by: age, SAPS II, reason for mechanical ventilation, variables associated with treatment (sedation, neuromuscular blockers, ventilation parameters), complications during mechanical ventilation (ARDS, sepsis, pneumonia, cardiovascular failure, kidney failure, liver failure, hematologic failure) and days on mechanical ventilation.
ARDS, acute respiratory distress syndrome; CI, confidence interval.
The practice of mechanical ventilation in the Mexican ICUs that participated in 4 international studies on mechanical ventilation conducted between 1998 and 201611–13 has changed over time, according to the scientific evidence available. The biggest change operated here has been a reduced programmed tidal volume. Although there is a tendency toward a lower mortality rate, no significant differences were seen between the first to the last study.
A significant finding that could in part account for the absence of important changes in the mortality observed is the differences in the characteristics of the patients included in the 4 studies, above all in the reason why mechanical ventilation was started in the first place. It has gone from a prevalence of patients with postoperative respiratory failure and trauma patients seen in 1998 to the fact that, in 2016, 1 of every 3 patients included suffered from neurological disease. This finding, the population included in our study and the progression of the most common diseases, is consistent with other series published including patients from a specific country15–21 or from several different geographical regions.11,22,23
We should mention here the low incidence of patients with exacerbated chronic obstructive pulmonary disease (COPD). However, in this case the situation of the Mexican ICUs is similar to what has been reported worldwide. In the remaining countries that participated in the international studies on mechanical ventilation11–14 this type of disease has gone down from 10% to 7% over a period of 18 years. This finding seems to be explained by the low incidence of COPD in Mexico as confirmed by the PLATINO study.24 In this study conducted with a general population of ≥40 years, the percentage of patients with COPD-like clinical criteria in Mexico City was 7.8% (almost half of the percentage of patients found in 4 other South American capitals included in the study). The factors cited by the authors to explain these findings are the altitude in Mexico City (the majority of the ICU that participates in our study are located in this city) and ethnical aspects. However, these are hypotheses that require further analysis.
Research on mechanical ventilation over the last 2 decades has been changing the routine clinical practice regularly.12 Nonetheless, there is still great heterogeneity on this regard.25
One of the changes would be greater use of NIV as the first attempt of ventilatory support. Although to this day there are few studies available that have analyzed this evolution, a study conducted in France reported an increase from 16% in 1997 to 24% in 2011.1 In our study, that started from a low use of NIV, a reduction was seen. A possible explanation may be the case-mix of our population: a low percent of patients with COPD and heart failure in whom NIV has its main indication. Also, a high percentage of patients with neurological disease in whom NIV has not proven beneficial. On the other hand, most of the hospitals that participated in the study are public hospitals where the pressure sustained by the healthcare system is very high and the demand for ICU beds is much higher. This leads to a constant lack of ICU beds. This difficulty to accept patients in the ICU has triggered the development of NIV in the ER area and in some hospital wards like pneumology and internal medicine units.
Probably, the greatest change induced by clinical research is the so-called lung-protective ventilation strategy that ventilates with low tidal volumes (<6mL/kg of the ideal weight) and high PEEP with the objective of maintaining the plateau pressure <30cmH2O. This strategy, initially indicated for patients with ARDS2,3 has been used in the general population of patients on mechanical ventilation.10 In general, in the epidemiologic studies on mechanical ventilation published so far, we can see a progressive reduction of the programmed tidal volume. In the studies conducted during the first years of this century15,16,18,22 tidal volume was between 8 and 10mL/kg. Studies published more recently,19,26 have reported a progressive reduction to tidal volumes <8mL/kg, but >6mL/kg while keeping plateau pressure within safe limits. In our study, the evolution was similar in patients with ARDS criteria and without ARDS: the programmed tidal volume gradually dropped to a median of 6mL/kg in 2016.
The lowest compliance with the recommendations is showed by the programmed PEEP level, that remains at levels <10cmH2O in all the studies analyzed. In our series, we saw a significant change in patients with ARDS, in whom a median of 10cmH2O was reached in the last cohort included. These changes reduced the driving pressure to levels that have been associated with higher survival rates in patients with ARDS.27
Another aspect of mechanical ventilation that has changed significantly over the last 20 years is weaning.28 Changes can be summarized in the early identification of those patients who can be weaned from mechanical ventilation, running a test to check the tolerance to spontaneous ventilation and, in patients with difficult or prolonged weaning, withdrawals with a daily spontaneous breathing test29 or gradual reduction of support pressure.30 Although all these changes are well supported by scientific evidence, there are very many changes in the routine clinical practice.
A survey31 conducted among 1144 intensivists from 6 different geographical areas (Canada, India, the United, Europe, Australia/New Zealand and the United States) reported a daily identification rate of patients who meet the criteria for starting weaning of around 70% and 96%, support pressure as the preferred method for the first spontaneous breathing trial (between 57% and 72% as opposed to 9–59% that prefer the T-tube), and the weaning of patients with difficult/prolonged weaning (31% to 72% of the responders). In our study, we saw significant clinical changes, some of which match the results reported by the survey. Therefore, in time, it is possible to see a preference for the use of support pressure both for the first spontaneous breathing trial and for difficult weaning. An important piece of information here is the high use of SIMV (with or without support pressure) for weaning (26% of the patients in 2016 were weaned using this method) despite the fact that clinical trials show that this method is the least recommended of all.29,30 In the above-mentioned survey31 it was reported that SIMV is rarely used as a weaning method, although there are regions such as India and Australia/New Zealand where the percentage is closer to the figures confirmed by our study.
The mortality rate recorded in our series was lower than the one reported in similar epidemiologic studies,18,32 probably due to differences in the populations included. The lack of a significant reduction of mortality over the study period may be explained by the relatively low mortality rate seen over the 1998 period (15%). That is why the changes seen in the baseline characteristics of the population included during the study periods could counteract the possible benefit of the changes seen in the management of mechanical ventilation such as the protective ventilation strategy for example. As a matter of fact, since the 2004 period there has been a non-significant reduction of mortality that became more pronounced until 2016 that could be associated with the progressive increase of the protective ventilation strategy.
Our study has several limitations, which should be considered when interpreting the data available. For example, the different sample size included in each cohort can bring about imprecision in some comparisons and estimations. Also, the participating ICUs were not exactly the same in all the studies. This means that there could be a factor associated with variability in the clinical practice that was not possible to assess.
In conclusion, the clinical management of mechanical ventilation in Mexican ICUs has been modified over a period of 18 years. The most significant change is the adoption of a ventilation strategy based on low tidal volumes to maintain a safe plateau pressure. These changes have not been associated with significant changes in mortality, which should be associated with mechanical ventilation but, also, with factors that this study did not analyze.
Authors/collaboratorsMCM, JE, AVG, UC, MP, IP, JRS, and EM were the coordinators of the international study on mechanical ventilation in Mexico during the different periods and participated in the recruitment stage of the participants. They also collaborated with the writing of the manuscript. AM, OP, and FFV collaborated with the statistical design and analysis of the study and participated in the writing of the manuscript as well. MCM, FFV, OP, and AE collaborated as intellectual consultats of the study and participated in its design, data mining, database analysis, and in the writing of the final version of the manuscript.
FundingNone.
Conflicts of interestNone declared.
We wish to thank all the local researchers of every participating Mexican ICU from the VENTILA Group.
F. Aguilera Almazán (Hospital General Regional n° 1, Chihuahua), M. Benítez Cortázar (Hospital Universitario de Puebla, Puebla), R. Carrillo Speare (Hospital PEMEX Sur, Mèc)xico DF), R. Castaño (Hospital de Cardiología del CNM, Mèc)xico DF), R. Corral (Hospital Excel, Tijuana, Baja California), D. N. D́Ector Lira (Hospital Metropolitano, Mèc)xico DF), G. Díaz Polanco (Hospital de Traumatología, Magdalena de las Salinas, Mèc)xico DF), J. J. Elizalde (Hospital ABC, Mèc)xico DF), R. Envila Fisher (Hospital Morelos, Chihuahua), G. Franco (Hospital General de Mèc)xico, Mèc)xico DF), P. García Balbuena (Hospital General Fernando Quiroz, Mèc)xico DF), O. Gayoso Cruz (Hospital Regional Adolfo Pèc)rez Mateos, Mèc)xico), L. Green (Instituto Nacional de Cancerología, Mèc)xico DF), J. O. Herrera Hoyos (Centro Mèc)dico Las Amèc)ricas, Mèc)rida), J. Hinojosa (Hospital Angel Leaño, Guadalajara), J. Huerta (Clínica Londres, Mèc)xico DF), V. A. Juárez (Hospital Santelena, Mèc)xico DF), M. Loera (Hospital General de Durango, Durango), C. López Alzate (Clínica del Mar, Mazatlan), E. López Mora (Instituto de Cardiología, Mèc)xico DF), S. Martínez Caro (Hospital Hidalgo Aguascalientes, Aguascalientes), R. Mèc)ndez Reyes (Hospital Regional 10 de Octubre, Mèc)xico DF), M. Mendoza (Hospital General de la Villa, Mèc)xico DF), O. Narváez Porras (Instituto Nacional de Enfermedades Respiratorias, Mèc)xico DF), E. Ortiz (Hospital General Irapuata, Guanajuato), A. Padua (Hospital General Torreón, Coahuila), M. Poblano (Hospital Juárez. Mèc)xico DF), V. Pureco Reyes (Hospital Regional 20 Noviembre, Mèc)xico DF), W. Querevalum (Hospital Central Cruz Mexicana, Mèc)xico DF), A. Quesada (Hospital Ntra. Sra. de la Salud, San Luis Potosí), A. Ramírez Rivera (Hospital de Enfermedades Cardiovasculares y del Tórax, IMSS, Monterrey), A. Tamariz (Hospital Clínica Centro, Chihuahua), A. Tamariz (Hospital Central Universitario, Chihuahua), A. Vargas (Hospital General de Pachuca, Pachuca), C. Vázquez (Hospital General Celaya, Guanajuato).
Researchers from the second study (2004)
J. J. Elizalde (Hospital ABC, Mexico City), P. Cerda (Centro Mèc)dico de Las Amèc)ricas, Mèc)rida) R. Mercado (Hospital Universitario de Monterrey, Monterrey), J. Albe Castañón (Instituto Mexicano del Seguro Social HECMNS XXI, Mexico City).
Researchers from the third study (2010)
A. J. Villagómez Ortiz (Hospital Regional 1.° de Octubre ISSTE, Mexico City), C. Cruz Lozano (Hospital Regional de PEMEX, Mexico City), Z. Maycotte Luna (Hospital Ángeles de las Lomas, Mexico City), F. López Bacal (Hospital Regional de Zona 1 del IMSS, Mexico City), J. J. Elizalde (Instituto Nacional de Ciencias Mèc)dicas y Nutrición Salvador Zubirán, Mexico City), G. Cueto Robledo (Hospital General de Mèc)xico, Mexico City), M. A. Treviño Salinas (Hospital Universitario de Nuevo León Dr. Eleuterio González, Nuevo León), R. Martínez Zubieta (Hospital Español de Mèc)xico, Miguel Hidalgo), C. Olvera-Guzmán y Marco Montes de Oca (Centro Mèc)dico ABC, Mexico City), S. A. çamendys-Silva (Instituto Nacional de Cancerología, Mexico City), J. S. Martínez Cano (Centenario Hospital Miguel Hidalgo, Aguacalientes), J. A. Baltazar Torres (Umea Hospital de Especialidades, Dr. Antonio Fraga Mouret, Mexico City), G. Morales Muñoz (Hospital Regional de Alta Especialidad de la Mujer, Villahermosa), A. Villa Delgado (Hospital Mèc)rida Yucatán, Mèc)rida), J. Ladape Martínez (Hospital Juárez de Mèc)xico, Mexico City).
Researchers from the fourth study (2016)
A. Ortega Pèc)rez (Centro Mèc)dico Lic. Adolfo López Mateos, Toluca), A. Chávez Morales (Hospital General de Mèc)xico Dr. Eduardo Liceaga, Mexico City), A. García Luna (Hospital Ángeles, León), A. Rugiero Cabrera (Hospital ABC Santa Fe, Mexico City), A. Rugerio Cabrera (Hospital ABC Observatorio, Mexico City), Á. A. Pèc)rez-Calatayud (Hospital General de Mèc)xico Dr. Eduardo Liceaga, Mexico City), A. Arellano (Hospital Regional de Alta Especialidad, Ixtapaluca), A. Velasco Gutièc)rrez (Hospital La Victoria, Cancún), A. J. Longino Gómez, Antonio Tamariz Becerra Álvarez Calderón (Hospital Ángeles, Chihuahua), R. Álvarez Calderón Christus Mugerza (Hospital Betania, Puebla), C. I. Reynoso Estrella (Hospital Civil de Guadalajara, Guadalajara) D. Dèc)ctor-Lira Espindola-Cruz (Centro Mèc)dico Dalinde, Mexico City), D. Gutièc)rrez-Zárate (Hospital Español, Mexico City), D. Esmeralda (Hospital Central Universitario, Chihuahua), E. D. Anica Malagón (Hospital General de Mèc)xico Dr. Eduardo Liceaga, Mexico City), E. Monares Zepeda (Hospital San Ángel Inn, Universidad, Mexico City), E. Deloya (Hospital de Alta Especialidad San Juan del Rio, Querèc)taro), E. Manzo Palacios (Hospital Metropolitano, Mexico City), F. de Jesús Montelongo (Hospital General Ecatepec Las Amèc)ricas, Mexico City), F. J. Flores Mejía, M. Ramírez Cervantes (Hospital General de Zona n.° 1, IMSS, Tepic Nayarit), G. Camarena Alejo (Hospital ABC Santa Fe, Mexico City), G. Vázquez de Anda (Centro Mèc)dico Nacional Siglo XXI, Mexico City), H. Vázquez (Hospital General de Zona n.° 1 del IMSS, La Paz), C. Larios Luna (Hospital de Especialidades del CMM Manuel Ávila Camacho del IMSS, Puebla), G. Morales Muñoz (Hospital Regional de Alta Especialidad de la Mujer, Villahermosa), S. E. Zamora (Hospital Juárez de Mèc)xico, Mexico City), G. Magaña Solano (Instituto Nacional de Neurología y Neurocirugía MVS, Mexico City), J. I. Sánchez González (Unidad Mèc)dica Alta Especialidad UMAE 34 IMSS, Monterrey), J. Rosendo Sánchez Medina (Hospital Regional de Ciudad Madero PEMEX, Tamaulipas), J. J. Zaragoza (Hospital Ángeles Acoxpa), J. A. Buensuseso Alfaro (Hospital Costamed Playa del Carmen, Quintana Roo), J. C. Dávila Fernández (Hospital General Zona n.° 1, IMSS, Oaxaca), J. C. Mijangos-Mèc)ndez (Hospital Civil de Guadalajara, Guadalajara), M. Martínez Medina (Hospital General de Zona n.° 5 IMSS, Nogales), M. Chacón Gómez (INR, CENIAQ, Mexico City), M. V. Calyeca Sánchez (Centro de Especialidades Mèc)dicas UCI Polivalente, Veracruz), J. J. Martínez Soria (Hospital General Irapuato, Irapuato, Guanajuato), R. Mèc)ndez Reyes (Hospital Regional 1.° de Octubre ISSSTE, Mexico City), R. J. García Graullera (Hospital Galenia, Cancún), R. Rosas (PEMEX, Villahermosa), S. Sanjuana Gómez Flores (Hospital Rubèc)n Leñero, Mexico City), S. Reyes Inurrigarro (Hospital General de Cholula, Puebla), S. Reyes Inurrigarro (UMAE Hospital de Traumatología y Ortopedia, Puebla), S. A. çamendys-Silva (Instituto Nacional de Cancerología, Mexico City), S. A. çamendy-Silva (Fundación Clínica Mèc)dica Sur, Mexico City), L. A. Sánchez Hurtado (Hospital de Especialidades Dr. Antonio Fraga Mouret Centro Mèc)dico Nacional La Raza, Mexico City), L. L. Villegas Castellanos (Hospital General Ajusco Medio, Mexico City), L. Zalatiel Maycotte (Hospital Texcoco, Texcoco), A. Estrada Gutièc)rrez (Hospital General de la Mujer SSM, Michocan), M. A. León (Hospital de Especialidades CMN S. XXI, IMSS, Mexico City).
Please cite this article as: Marín MC, Elizalde J, Villagómez A, Cerón U, Poblano M, Palma-Lara I, et al. ¿Se han producido cambios en la aplicación de la ventilación mecánica en relación con la evidencia científica? Estudio multicéntrico en México. Med Intensiva. 2020;44:333–343.