To describe the high-flow nasal cannula (HFNC) indications in the Spanish pediatric critical care units (PICUs).
DesignDescriptive cross-sectional observational study.
SettingElectronic survey among members of the Spanish Society of Pediatric Intensive Care (SECIP). It was sent weekly from April 10, 2023, to May 21, 2023.
ParticipantsAll SECIP members.
InterventionsNone.
Main variables of interestThe questions focused on workplace, years of experience, use or non-use of HFNC, justification and expectations regarding its application, starting point within each center, clinical criteria for indication, existence of clinical guidelines, evaluation during its use, and criteria and mode of withdrawal.
ResultsTwo hundred and two participants, 176 were from Spain. Of these, 87/176 had over ten years of experience. One hundred sixty two use HFNC and 66/162 have HFNC clinical guidelines. Acute bronchiolitis (138/162) and respiratory assistance after extubation (106/56) are the two main indications. For 62/162 HFNC may reduce therapeutic escalation. Neuromuscular diseases (105/162) and anatomical airway diseases (135/162) are the two main contraindications. The reasons to do not use HFNC were the absence of evidence about it effectiveness (8/14) and its inadequate cost/effectiveness balance (8/14).
ConclusionsA majority of Spanish pediatric intensivists use HFNC. Its application and withdrawal appears to be primarily based on clinical experience. Besides, those who use HFNC are aware of its limitations and the lack of evidence in some cases. It is necessary to develop single-center and multicenter studies to elucidate the effectiveness of this therapy in the context of critically ill children.
Describir las indicaciones de las cánulas nasales de alto flujo (CNAF) en las unidades de cuidados intensivos pediátricos (UCIP) españolas.
DiseñoEstudio observacional descriptivo transversal.
ÁmbitoEncuesta electrónica a miembros de la Sociedad Española de Cuidados Intensivos Pediátricos (SECIP). Se envió semanalmente desde el 10 de abril de 2023 hasta el 21 de mayo de 2023.
ParticipantesMiembros de la SECIP.
IntervencionesNinguna.
Variables de interésprincipalesLas preguntas se centraron en lugar de trabajo, años de experiencia, uso o no de la CNAF, justificación y expectativas respecto a su aplicación, criterios clínicos de indicación, existencia de guías clínicas, la evaluación durante su uso y los criterios de retirada.
ResultadosDoscientos dos participantes, 176 españoles. De ellos, 87/176 con más de diez años de experiencia. Ciento sesenta y dos usan CNAF y 66/162 tienen guías clínicas. La bronquiolitis aguda (138/162) y la asistencia respiratoria tras la extubación (106/56) son las indicaciones principales. Para 62/162 la CNAF puede reducir la escalada terapéutica. Las enfermedades neuromusculares (105/162) y las alteraciones anatómicas de la vía aérea (135/162) son las principales contraindicaciones. Las razones para no utilizar la CNAF fueron ausencia de evidencia sobre su efectividad (8/14) e inadecuado balance costo/efectividad (8/14).
ConclusionesLa mayoría de los intensivistas pediátricos españoles utilizan CNAF. Su aplicación parece basarse en la experiencia clínica. Además, quienes utilizan CNAF son conscientes de sus limitaciones y de la falta de evidencia en algunos casos. Es necesario desarrollar estudios unicéntricos y multicéntricos para dilucidar la efectividad de esta terapia en el niño críticamente enfermos.
In recent years, the use of high-flow nasal cannula (HFNC) has become an extended therapeutic strategy in paediatrics.1 This phenomenon has also permeated the context of pediatric intensive care (PICU).2 However, this has occurred without solid scientific evidence, which seems to contradict the clinical perception of those who apply it. Therefore, at present, the use of HFNC in PICU is not only a subject of clinical research but also a cause for controversy and debate.3–4
The HFNC provides a continuous flow of humidified and heated air. It may reduce heat loss, washing out the dead space in the airway, providing positive end-expiratory pressure, and maintaining respiratory system humidity. Additionally, it allows the administration of high concentrations of oxygen, providing effective respiratory support and improving tissue oxygenation.5 HFNC does not require the use of face masks or other interfaces, which may reduce anxiety and stress in pediatric patients.6 All of this has led to its use occasionally being favored over other traditional non-invasive ventilation modalities.7
The use of HFNC in critically ill pediatric patients is not widely standardized. Its use is based on the experience of those who recommend it, despite the limited body of evidence beyond clinical recommendations and guidelines.4 This is also the case in our country, where the application of HFNC in different Spanish services has not been accurately described. Therefore, it seems necessary to carry out an analysis to understand not only the mode and reasons for those who recommend it but also the reasoning behind those who do not.8
For all these reasons, this article presents the results of an electronic survey conducted among pediatricians who are members of the Spanish Society of Pediatric Intensive Care (SECIP). A demographic study of the participants is conducted, indications for HFNC are described, as well as the prescribed mode of administration and the decisions made regarding its withdrawal. Furthermore, those who do not have access to this type of assistance are asked to justify their reasons for not using it.
Materials and methodsA descriptive cross-sectional observational study was conducted using an anonymous electronic survey that was not validated (Google Doc® questionnaire). The survey was sent weekly to members of the Spanish Society of Pediatric Intensive Care from April 10, 2023, to May 21, 2023. Completed questionnaires were later analyzed.
The questions were focused on the participants’ workplace, years of experience, use or non-use of HFNC, justification and expectations regarding its application, starting point within each center, clinical criteria for indication, existence of clinical guidelines, evaluation during its use, and criteria and mode of withdrawal. Descriptive analysis of the responses was performed using SPSS® 19 for Windows. The questions and possible responses are described in the Supplementary material 1.
ResultsDemographic characteristics of the participantsA total of 202 SECIP members responded to the survey, with 176/202 being from Spain and 26/202 from various countries in Central and South America. Only the responses from participants currently working in Spain are included in this analysis (Supplementary material 2).
Of these, 167/176 are involved in clinical work in Pediatric Intensive Care, with 87/176 having more than ten years of experience, 42/176 having four to ten years of experience, and 36/176 having three or fewer years of experience. Among the participants, 168/176 work in a Pediatric Intensive Care unit affiliated with a public university; while 5/176 work in a private university. The median number of beds in their workplace is 9, with a range of 6–16. When asked about the availability of HFNC in their service, 162/176 responded affirmatively. The annual number of admissions in the participants’ services is described in Supplementary material 3.
Indication of high-flow nasal cannula (HFNC)Commencing HFNC assistance requires mandatory admission to the Pediatric Intensive Care Unit (PICU) for 25 out of 162 participants. The location where this type of respiratory support can be initiated at the participants’ workplace was pediatric intensive care 140/162, pediatric emergencies care 105/162 and hospitalization ward 40/162.
When asked about the main mechanism of action of this therapy, 62 out of 162 participants stated that it is “Clearing the nasopharyngeal dead space”, 36 out of 162 participants mentioned “Improving lung compliance and gas conductance through warm and humidified air”, 32 out of 162 participants stated “Reducing respiratory effort through adequate airflow”, and 15 out of 162 participants mentioned “Providing positive pressure to improve lung distension”. The remaining 17 out of 162 participants offered different arguments.
Regarding the aspects in which HFNC appears superior to low-flow nasal cannula, the participants indicated the following: 1) Comfort 94/162; 2) Decreased need for therapeutic escalation 62/162; 3) No objective evidence indicating improvement in any of the above aspects 61/162; 4) ICU length of stay 19/162; 5) Hospital length of stay 18/162; and 6) Duration of oxygen therapy 9/162.
When questioning the superiority of HFNC over non-invasive ventilation, the responses were as follows: 1) No objective evidence indicating improvement in any of the above aspects 89/162; 2) Comfort 74/162; 3) Costs 29/162; 4) Decreased need for therapeutic escalation 15/162; 5) ICU length of stay 6/162; 6) Decreased intubation rate 4/162; and 7) Duration of oxygen therapy 4/162.
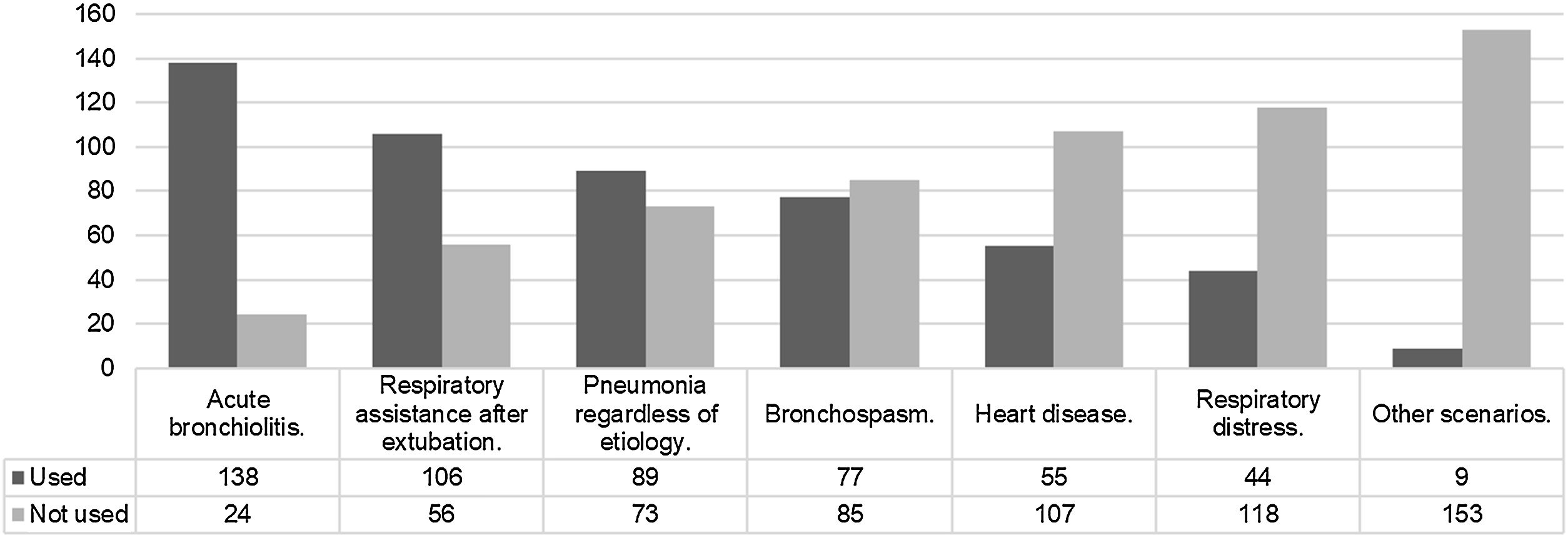
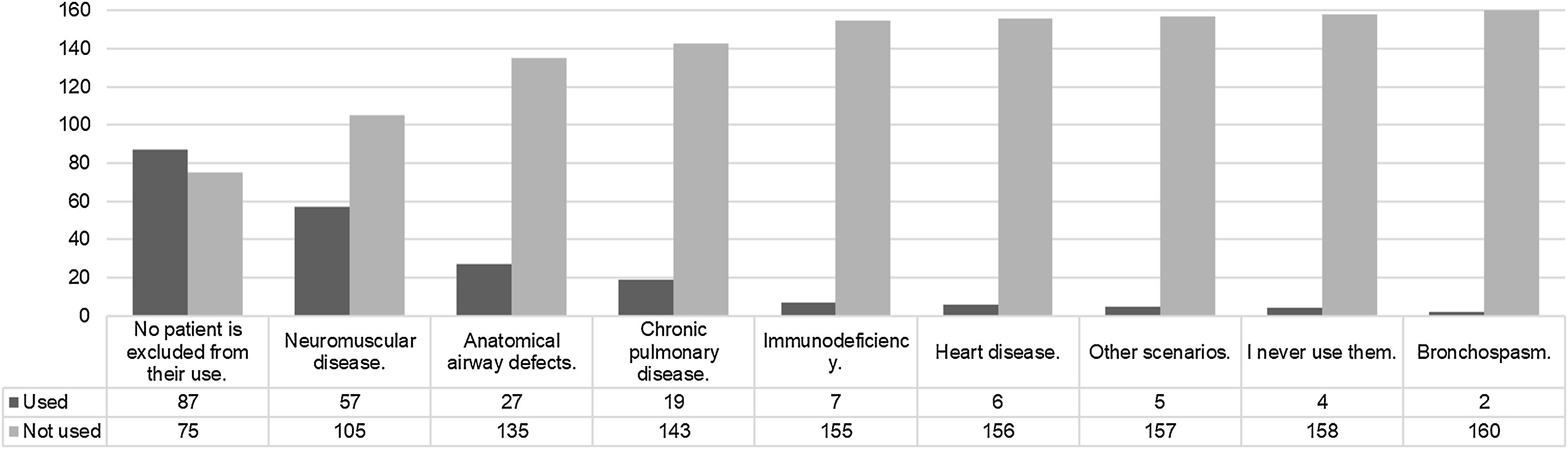
The clinical scenarios for which HFNC is indicated are described in Fig. 1. Meanwhile, Fig. 2 describes the scenarios for which the use of HFNC is never considered.
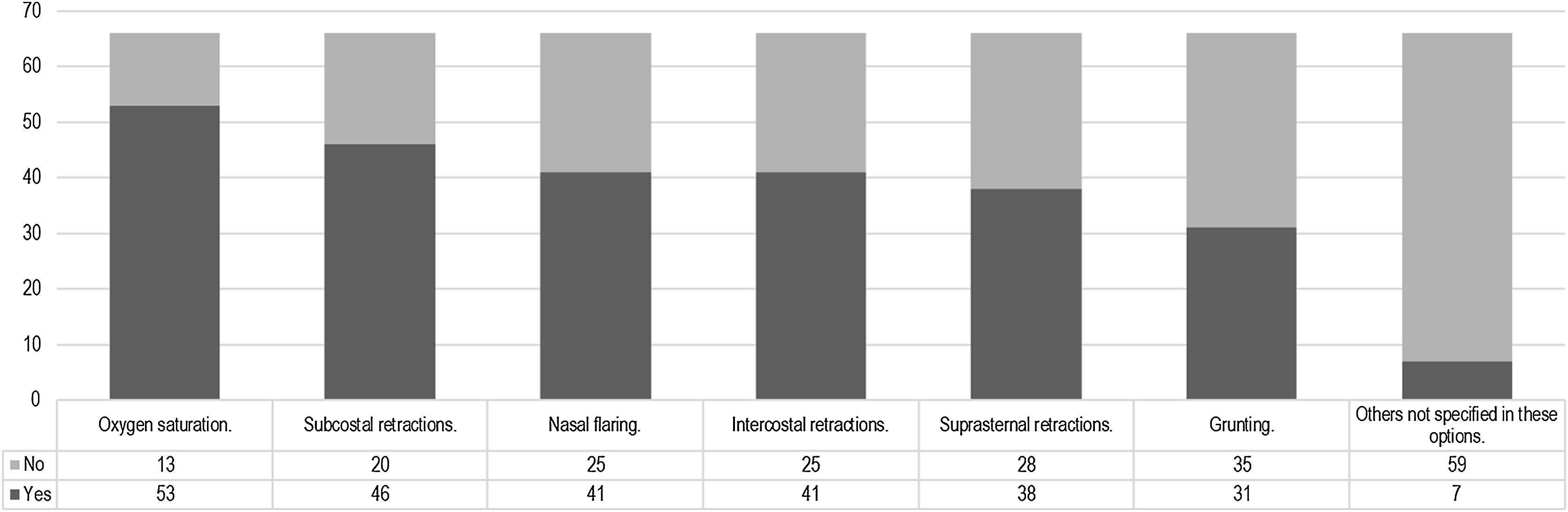
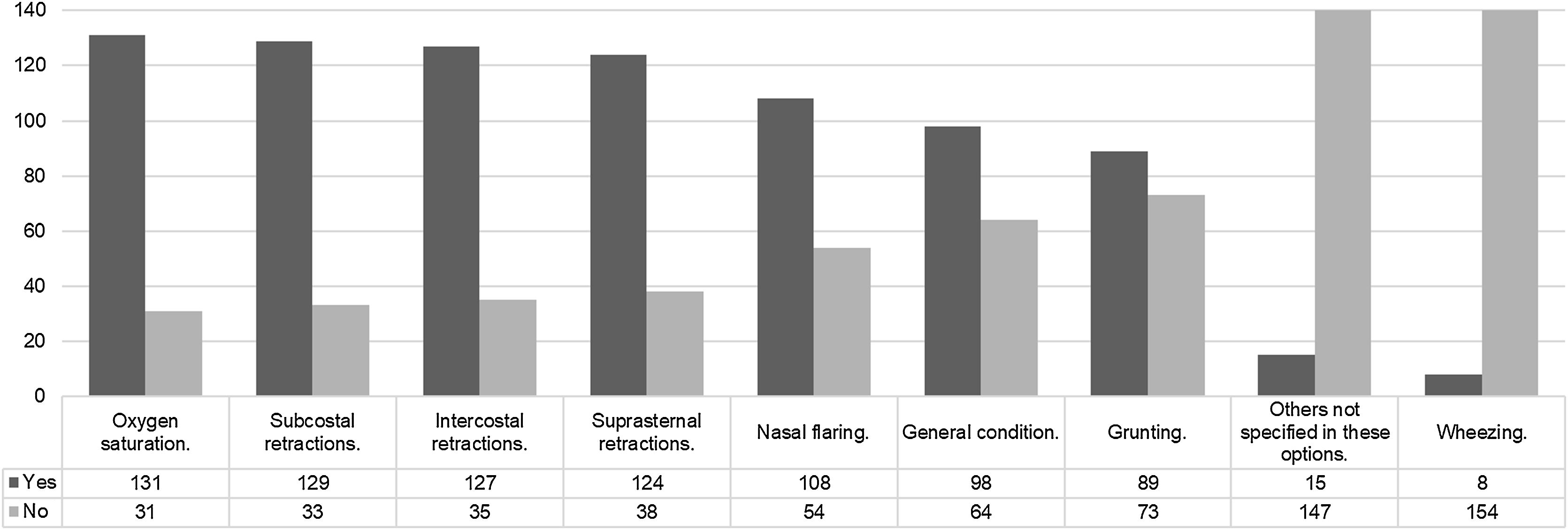
The use of high-flow nasal cannula (HFNC) in units with specific clinical guidelinesOut of the participants, 66 out of 162 have clinical guidelines for HFNC in their service. Among them, the guidelines are used “most of the time” in 43 out of 66 cases, “occasionally” in 14 out of 66 cases, and “always” in 2 out of 66 cases. The rationale for the development of the clinical guidelines is described in Supplementary material 4. The clinical signs considered for initiating HFNC can be observed in Fig. 3. Additionally, 12 out of 66 participants use specific scales, while 54 out of 66 participants use the usual scales for each disease. The “Bronchiolitis Score of Sant Joan de Déu” or BROSJOD was mentioned by 12 out of 66 participants. The development of clinical guidelines for HFNC was a collaborative work by pediatricians and nurses 35/66, based on clinical experience 24/66, based on a literature review 24/66. Ten of 66 did not know how the guidelines were done.
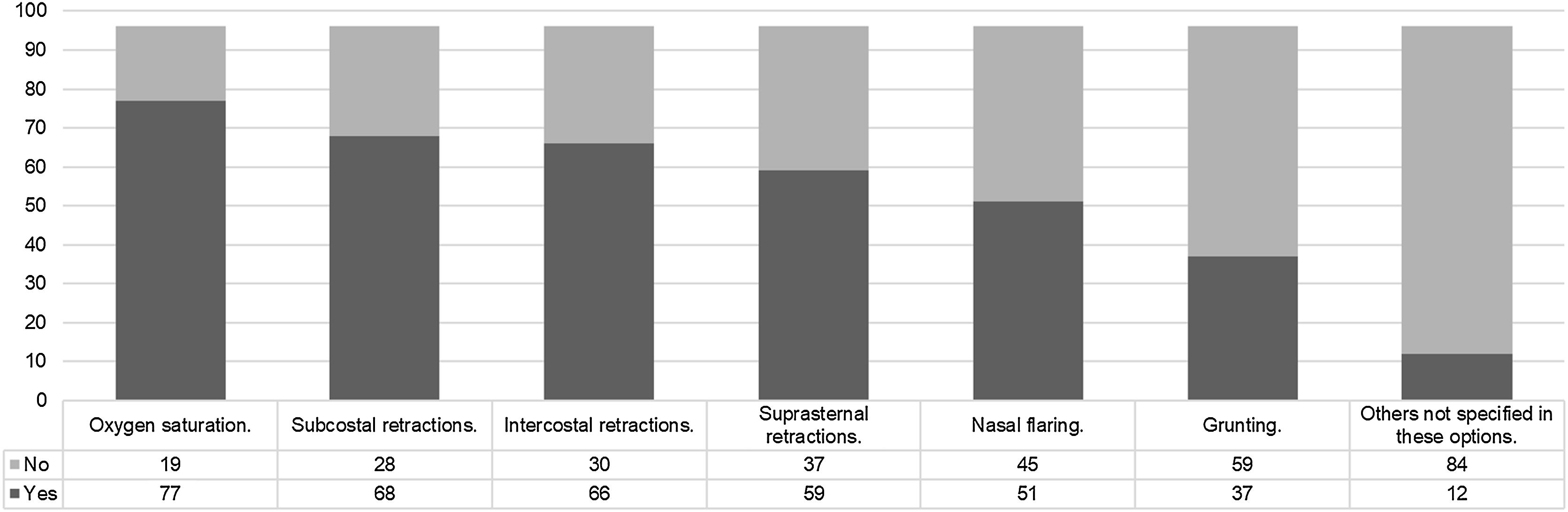
Usage of high-flow nasal cannula (HFNC) in units without specific clinical guidelinesThe clinical signs used to initiate HFNC by those who do not have specific clinical guidelines are described in Fig. 4.
Initiation and maintenance of high-flow nasal cannula (HFNC)The weight of the patient determines the initial flow for 116 out of 162 participants. Clinical criteria are used by 18 out of 162 participants, a protocol is followed by 13 out of 162 participants, age is considered by 7 out of 162 participants, and a combination of the above factors is used by 8 out of 162 participants. The use of a protocolized assessment of clinical response is indicated in Supplementary material 5. Regarding the performance of blood gas analysis after the initiation of HFNC the response is described in Supplementary material 6.
The responses about the signs used to assess the response to HFNC are summarized in Fig. 5. Regarding the maximum inspiratory fraction of oxygen considered in HFNC, it is individually assessed for 83 out of 162 participants. For 38 out of 162 participants, it is above 50%, for 27 out of 162 participants, it is above 60%, for 11 out of 162 participants, it is above 30%, and for 3 out of 162 participants, it is above 80%.
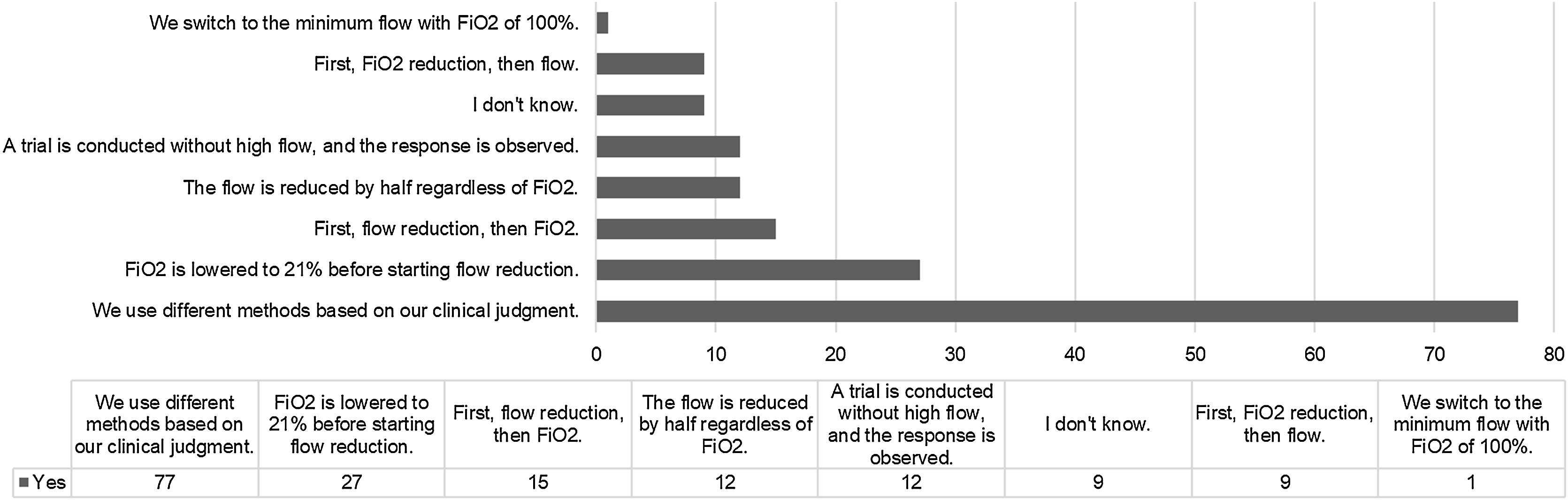
Withdrawal of high-flow nasal cannula (HFNC)Among the participants, 118 out of 162 do not have clinical guidelines for the withdrawal of HFNC. Among those who have guidelines, 25 out of 44 use them “most of the time”, 11 out of 52 use them “occasionally”, 6 out of 44 use them “rarely”, 8 out of 44 never use them, and 4 out of 44 always use them. When asked about the method used to discontinue the use of HFNC, the responses are described in Fig. 6.
Reasons for the unavailability or lack of indication for HFNCAmong the participants, 14 out of 176 indicated that they do not have HFNC available in their service. The reasons for this were: “It does not prevent clinical worsening in patients where it is applied” for 8/14, “It incurs increased costs at the expense of low or inadequate effectiveness” for 8/14, “The potential delay of other appropriate respiratory approaches poses a risk for 10/14 and “there is no clinical evidence of its benefit in critically ill pediatric patients” for 12/14.
DiscussionThis study shows that most of Spanish pediatric intensivists have access to HFNC in their clinical practice. The use of this therapy, which can also be initiated in other hospital departments, does not typically require mandatory admission to the Pediatric Intensive Care Unit (PICU). HFNC is applied in multiple clinical conditions, with neuromuscular disease being the most common contraindication. Comfort and its impact on reducing the need for therapeutic escalation are among the common reasons for its indication. However, there are still debates about its objective benefits and the potential associated costs. Furthermore, the existence of clinical guidelines for the initiation of HFNC is not widespread, and individualization based on clinical criteria is prioritized when discontinuing the therapy.
Compared to other previously published surveys on HFNC in the context of critically ill children, the number of respondents in this study was high.1–9 At the time of writing this text, the Spanish Society of Pediatric Intensive Care (SECIP) had 588 members, and 176 Spanish intensivists participated in the survey. Participants whose clinical work is not carried out in Spain were excluded to focus the observations on our specific context. As reflected in the results, a large majority of Spanish pediatric intensivists seem to have access to HFNC in their services. Only one out of ten respondents reported not having access to HFNC, and the reasons for this include the absence of evidence, potential delay of other therapies, or high costs.8–12
The profile of the participants reveals an average experience of over four years and a strong affiliation with university hospitals. It is noteworthy that the surveyed participants work in departments with around ten beds and an estimated annual admission rate exceeding three hundred in more than half of the participants. These combined aspects are of particular interest as they undoubtedly reflect the opinions of professionals with significant clinical experience.
It is observed that HFNC can be initiated beyond the Pediatric Intensive Care Unit (PICU). Almost two-thirds of the participants reported that pediatric emergency services in their centers have and use this therapy.13,14 However, this does not necessarily require admission to the PICU.15 We did not ask about the HNFC system used in each center. This could influence the possibility of initiating it beyond PICU. It is likely that the indication for HFNC aims to reduce the likelihood of admission to intensive care units.8 This particular aspect was not addressed in this study.
Considering the HFNC mechanism of action, there is observed disparity in criteria. This variability is simply a reflection of the different theoretical foundations that support this clinical approach and justify the range of possible indications. When asked about its superiority over low-flow nasal cannula, comfort, and the potential to limit therapeutic escalation are identified as key factors. It is worth noting that both considerations are often made with the understanding that current evidence is limited. In this regard, clinical experience and individualization may play a crucial role.
When asked about its value compared to non-invasive ventilation, it is observed that at least half of the participants do not consider it superior. Comfort is the main reason for its indication, while responses indicating a real clinical benefit of HFNC over non-invasive ventilation are in the minority. Participants, through their responses, reflect that HFNC is not an alternative ventilation modality to non-invasive ventilation (the philosophy does not seem to be “either/or”).16 Understanding its limitations, HFNC is presented as a therapeutic possibility for selected patients.7
Thus, when asked about the possible indications of HFNC, acute bronchiolitis,17 post-extubation support,18 pneumonia, and bronchospasm19,20 are clinical scenarios in which HFNC is applicable.4–21 In the adult population, there are studies with both positive and negative results, while in the pediatric population, objective data appears to be limited and inconsistent.22–24 Evaluating indications from the other perspective, namely pure contraindications, it is interesting to observe that HFNC loses relevance in neuromuscular diseases or airway defects. While indications are broad and subject to variability, contraindications tend to be clearer.25
Regarding the existence of clinical guidelines, these have often been developed in collaboration with the nursing service, which is logical considering that the success of various respiratory therapies relies heavily on the work of these professionals. The absence of clinical guidelines for HFNC may contribute to variability and, at the same time, place greater importance on the clinician’s judgment. In the absence of protocols, clinical expertise and experience come into play. However, the presence of guidelines does not seem to be a differentiating factor in determining the signs used to initiate HFNC (as shown in Figs. 3 and 4).
Regarding the prescribed flow rate, it can be affirmed that weight is the most commonly used objective data. This is accompanied by a prevalent protocolized assessment that is usually not followed by post-treatment blood gas analysis. Additionally, multiple clinical criteria are used to evaluate the response (Fig. 5), and it is accompanied by an individual and strict assessment of oxygen requirements. In fact, one in three participants sets the upper limit of tolerable inspired fraction of oxygen at 50% to assess the need for HFNC substitution. These facts are of particular interest since one of the main concerns associated with the use of HFNC is the potential delay in other respiratory interventions.26 Overall, it seems that once the therapy is initiated, frequent clinical monitoring is not only common but also aims to accurately assess the likelihood of non-response.27,28 Finally, during HFNC withdrawal, different methods are prioritized based on clinical judgment. Once again, individualization and experience play a crucial role. This aspect has been described by other authors and, as previously mentioned, appears to be a common approach in clinical practice with this therapy.
This study has significant limitations. Clinical data related to the use of HFNC are not described, only opinions based on clinical experience are presented. Additionally, selection bias may have occurred as those with a more favorable view towards the use of this therapy may have been more likely to respond. All of these factors make it challenging to draw conclusions about the effectiveness of HFNC and only allow for the formulation of working hypotheses.
In summary, it can be observed that most of Spanish pediatric intensivists have access to and use HFNC in their patients. The use and withdrawal of HFNC are primarily based on clinical experience and observation. However, those who use HFNC are aware of its limitations and the lack of evidence in some cases. Therefore, it is of great interest, based on studies like this, to consider the development of both single-center and multicenter studies that can elucidate the reasons and purpose of this therapy in the context of critically ill children.
Authors’ contributionsAGS and AMV designed the web questionary. AGS analyzed the data. AMV co-wrote and corrected it. AGS and AMV coordinated the work. VMiA acts as an internal reviewer. All authors read and approved the final manuscript.
Thanks to all members of the respiratory group of the Spanish Society of Pediatric Intensive Care who participated in this study. To all PICU professionals in Spain who participated in the management of the patients included in this study.
The following are Supplementary data to this article: