Right ventricular dysfunction is common in critically ill patients, and is associated with increased mortality. Its diagnosis moreover remains challenging. In this review, we aim to outline the potential mechanisms underlying abnormal biomechanics of the right ventricle and the different injury phenotypes. A comprehensive understanding of the pathophysiology and natural history of right ventricular injury can be informative for the intensivist in the diagnosis and management of this condition, and may serve to guide individualized treatment strategies.
We describe the main recommended parameters for assessing right ventricular systolic and diastolic function. We also define how to evaluate cardiac output and pulmonary circulation pressures with echocardiography, with a focus on the diagnosis of acute cor pulmonale and relevant applications in critical disorders such as distress, septic shock, and right ventricular infarction.
La alteración del ventrículo derecho (VD) es frecuente en los pacientes críticos y su disfunción se asocia a mayor mortalidad, lo que plantea un reto clínico en su diagnóstico. En esta revisión, pretendemos describir los posibles mecanismos de la biomecánica anormal del VD y los distintos fenotipos de su lesión. La comprensión de la fisiopatología y la historia natural de la lesión del VD puede informar al intensivista sobre el enfoque del diagnóstico y la monitorización de este, así como sobre la aplicación de intervenciones personalizadas con relevancia terapéutica.
Se realiza una descripción de los parámetros de evaluación de la función sistólica y diastólica del VD, junto con la estimación del gasto cardiaco y las presiones del circuito pulmonar mediante ecocardiografía, con énfasis en el diagnóstico del cor pulmonale agudo junto con aplicaciones clínicas en el paciente crítico como en el distrés, shock séptico e infarto de VD.
Many disease conditions in critically ill patients have an impact on the function of the right ventricle (RV), and dysfunction of the latter is associated with increased patient mortality.1 This disorder may result from contractility alterations secondary to coronary ischemia or sepsis, or increased afterload as in acute respiratory distress syndrome (ARDS) or pulmonary thromboembolism (PTE) – though it may also be a consequence of the treatments used, such as vasoactive drugs, mechanical ventilation (MV) or water overload.1–4 Understanding the pathophysiology and natural history of RV injury and adequate on-site monitoring can help the intensivist establish a diagnosis and prescribe treatment to optimize RV function.1,5
Ultrasound is a noninvasive and widely available technique that has become a key tool for routine clinical evaluation purposes.6–8 Right ventricle alterations are common in the critical patient, though their diagnosis may prove challenging and requires physiological knowledge of cardiorespiratory diseases in order to offer adequate treatment.5
Pathophysiology of RV dysfunction, its importance in intensive care, and ventricular interdependenceThe RV is semilunar in shape and envelops the left ventricle (LV). It consists of three segments: inflow tract, body and outflow tract. The end-diastolic volume of the RV is slightly greater than that of the LV, and as a result, it has a slightly lesser ejection fraction (RVEF). The helicoid distribution of its muscle fibers generates a mainly longitudinal contractile pattern, due to the lesser amount of circumferential fibers. The RV wall is also comparatively thinner (under 5 mm) and is characterized by great distensibility and poor tolerance of increased pressure in the pulmonary circuit.9–11 The chronic increase in RV afterload generated by pulmonary hypertension (PHT) produces an increase in the thickness of the RV wall as a compensatory mechanism.
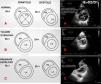
Both ventricles are anatomically and physiologically integrated through the interventricular septum. Ventricular interdependence is defined as the changes produced in one ventricle secondary to an increase in pressure or volume in the other, and is evaluated along the short axis of the parasternal plane.12 Under normal conditions, the IVS is concave towards the LV throughout the cardiac cycle, and the eccentricity index (EI) of the LV is 1. This index is defined as the ratio between the anteroposterior and septolateral diameters of the LV (Fig. 1A).13 When the RV experiences volume overload, it undergoes dilatation accompanied by flattening of the IVS, with EI > 1 during diastole (Fig. 1B). Excessive preload may cause impaired contractility and a decrease in coronary perfusion pressure.1 However, in the presence of pressure overload, the RV experiences dilatation with septal deviation towards the LV and EI > 1 during both systole and diastole, with a “D”-shaped LV, indicative14 of a poor patient prognosis (Fig. 1C).15 This finding is known as the reverse Bernheim effect.9,12
Ventricular interdependence. RV: right ventricle; LV: left ventricle; EI: eccentricity index; A: normal eccentricity pattern of the LV; B: volume overload with flattening of the septum only in diastole; C: pressure overload with flattening of the septum in systole and diastole; D2: anteroposterior diameter of the LV; D1: septo-lateral diameter of the LV.
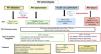
There is no universally accepted definition of RV failure.4,5 Recently, three different phenotypes have been identified in patients with ARDS (normal RV function, RV dilatation, and dilatation of the RV with impaired systolic function), associated with different clinical outcomes16,17 (Fig. 2). Dilatation of the RV causes expansion of the tricuspid valve annulus and the appearance of tricuspid insufficiency (TI), which produces venous congestion and associated renal and/or hepatic damage, with an increase in mortality.18 Congestion should be evaluated based on the size of the inferior vena cava (IVC) and its variation during the breathing cycle, together with the hepatic venous flow pattern.
RV phenotypes. RV: right ventricle; LV: left ventricle; RV/LV: right and left ventricle end-diastolic area ratio; TAPSE: tricuspid annular plane systolic excursion; PA: pulmonary artery; IVS: interventricular septum; Tac: pulmonary artery acceleration time; PAPs: pulmonary artery systolic pressure; CVP: central venous pressure; IVC: inferior vena cava; CVVH: continuous venous-venous hemodiafiltration.
The interaction between the RV and the pulmonary artery (PA) circulation under different loading conditions is referred to as right ventricle – pulmonary artery (RV-PA) coupling and determines the relationship between RV contractility, measured by end-systolic elastance (Ees), and PA afterload, measured by pulmonary artery elastance (Ea)1,18 (Fig. 2). The system is considered to be coupled when Ees/Ea > 1.19,20 Acute pulmonary vascular dysfunction due to multiple causes such as thrombosis, lung edema, MV and vasoconstriction (secondary to hypoxemia, hypercapnia, and acidosis) leads to acute PHT, which in turn induces an increase in the intrinsic contractile force of the RV to compensate the increase in afterload (homeometric adaptation or Anrep effect).21 This should be reported as a hyperdynamic RV, though in critically ill patients it may be limited by systemic infection and hypotension.22 Another mechanism is RV dilatation to preserve blood flow (heterometric adaptation or Starling mechanism)5,23 and reduction of Ees/Ea to < 1, caused by the increase in Ea, which can lead to RV-PA decoupling and a negative diastolic interventricular interaction, with a RV end-diastolic pressure (EDP) higher than that of the LV – which negatively affects LV filling and cardiac output.5,18
Quantification of the right ventricle chambersBecause of its anatomy, the RV must be examined using multiple acoustic windows, including the long parasternal axis, short parasternal axis (inflow tract and outflow tract of the RV), apical four-chamber (4C) window, the modified apical window for the RV, and the subcostal window, using transthoracic echocardiography (TTE).24,25 The report should present an evaluation based on qualitative and quantitative parameters,24 though in immediate patient life-threatening situations, there are protocols guided by qualitative parameters of great reproducibility and correlation versus other cardiac assessment techniques such as magnetic resonance imaging (MRI) and three-dimensional (3D) echocardiography,26,27 provided the exploration is performed by an expert operator.28Table 1 describes the echocardiographic parameters for the basic study of the RV.
Basic echocardiographic parameters in the study of the right ventricle.
| Ratio between area of RV and area of LV |
| IVS motion |
| RV wall thickness in subcostal plane |
| TAPSE |
| IVC size and collapse |
| VmaxTI |
RV: right ventricle; LV: left ventricle; IVS: interventricular septum; TAPSE: tricuspid annular plane systolic excursion; IVC: inferior vena cava; VmaxTI: maximum velocity of tricuspid insufficiency.
Although the plane corresponding to the short parasternal axis is the best option for evaluating ventricular interdependence, the apical 4C plane is the projection indicated for performing measurements of the size of the right atrium (RA) and the RV. Dilatation of the RV is based on the ratio between its transverse diameter or end-diastolic area (EDA) concerning the LV, measured in end-diastole. The normal ratio is RV/LV < 0.6, while 0.6–1 is indicative of moderate dilatation, and > 1 corresponds to severe dilatation.5,29,30 The quantification parameters are described in Table 2 and Fig. 3.
Echocardiographic parameters for quantification of the size and systolic function of the right ventricle.
| Parameter | Normal value | Characteristics |
|---|---|---|
| Wall thickness (mm) | < 5 | The lateral wall of the RV is measured in the subcostal planeThickness > 5 mm indicates RV hypertrophy and suggests pressure overload in the absence of other disease |
| RV/LV ratio | < 0.6 | 0.6–1 moderate RV dilatation>1 severe RV dilatation |
| Eccentricity index | = 1 | >1 in diastole suggests volume overload and >1 in systole and diastole indicates pressure overload |
| TAPSE (mm) | > 17 | Reproducible, easy to obtainGood correlation of RVEF with MRI, FAC and ECO 2DLimitation: angle dependent |
| FAC (%) | > 35 | Less reproducible, requires good 2D imageGood correlation with MRILimitation: quantification difficulty |
| S´ wave with TDI (cm/s) | > 9.5 | Reproducible, easy to obtainGood correlation with ECO 2D, MRILimitation: angle dependent |
| TEI index | RIMP > 0.43 via PW and > 0.54 via DTI | Limitation: with PW, measurement cannot be made in one same beat, with DTI measurement of IVRT and IVCT is made in the same beat |
| RV free wall strain (%) | > −20% | Requires high 2D image quality, avoid angulations of apical 4C windowLimitation: requires offline software for calculationMedium quantification difficulty |
| RV ejection fraction in 3D (%) | > 45% | Precise measurement of volumes and EFGood correlation with MRI, with slightly lesser volumesLimitation: few studies in critical patients |
| Speckle-tracking | Independent of Doppler angleRequires good 2D image quality, affected by cardiac motion artifacts in planeLimitation: quantification difficultyRequires software not readily available in critical care |
RV: right ventricle; LV: left ventricle; TAPSE: tricuspid annular plane systolic excursion; FAC: fractional area change; DTI: Doppler tissue imaging; PW: pulsed wave Doppler; MRI: magnetic resonance imaging; IVRT: isovolumetric relaxation time; IVCT: isovolumetric contraction time.
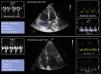
A. TAPSE. B. FAC. C. Tissue Doppler. The arrow indicates the systolic wave and the star indicates the velocity of the e wave. D. TEI index: measures the ratio between the isovolumetric contraction and relaxation times in relation to systolic ejection. It can be quantified with pulsed Doppler or from the tissue Doppler registry. The advantage of tissue Doppler is that it can record all the information of the cardiac cycle in the same beat, and improves the reproducibility of the technique. Measurement is made of the isovolumetric contraction time (ICT), the isovolumetric relaxation time (IRT) and the ejection time (ET) in the pulsed tissue Doppler spectrum of the lateral tricuspid annulus. E. Strain.
- -
M mode
- o
TAPSE (tricuspid annular plane systolic excursion). This parameter represents a measure of longitudinal function. It is determined in the apical 4C plane with the cursor optimally aligned in the lateral tricuspid annulus, and measures the degree of longitudinal displacement of the annular segment of the RV from end-diastole to the systolic peak. It is not particularly dependent upon good image quality. The TAPSE has a prognostic value in PHT,31 and a value of < 17 mm suggests systolic dysfunction of the RV.24 The parameter has limitations in post-heart surgery patients (Fig. 3A).
- -
Two-dimensional (2D) mode
- o
FAC (fractional area change). This parameter estimates global ventricular function. It is quantified in the apical 4C plane through tracing of the end-diastolic (EDA) and end-systolic area (ESA) of the RV, taking care to include the trabeculae in the cavity. Formula: (EDA-ESA)/EDA x 100, values < 35% indicate systolic dysfunction.24 The FAC is an independent predictor of heart failure, sudden death, stroke and mortality in patients with PTE.32 Compared with TAPSE, the FAC is more precise in estimating systolic function, taking MRI as reference33 (Fig. 3B).
- -
Doppler
- o
Doppler tissue imaging (DTI). Systolic pulse wave velocity (Sʹ). This parameter is obtained in the apical 4C plane using DTI, placing the cursor of the pulsed-wave (PW) Doppler at the middle portion of the basal segment of the lateral tricuspid annulus. It measures the longitudinal velocity. Although the parameter physiologically decreases with age, a value < 9.5 cm/s is considered to be abnormal24 (Fig. 3C).
- o
Right ventricle index of myocardial performance (RIMP) or Tei index. This is an index of the global performance of the RV. The isovolumetric contraction time (IVCT), isovolumetric relaxation time (IVRT) and ejection time (ET) should be measured from the same heartbeat using PW or DTI in the lateral tricuspid annulus. It is important to ensure that the non-consecutive beats have similar RR intervals. The index may be underestimated under conditions of high RA pressures, which will shorten IVRT. A RIMP > 0.43 via PW and > 0.54 via DTI indicates dysfunction of the RV24 (Fig. 3D).
- o
Strain/strain rate tissue deformation image. The data obtained from the DTI study can be used to determine the degree of myocardial deformation of the free wall (strain) and the velocity of myocardial deformation (strain rate). Strain and strain rate estimate the global and regional systolic function of the RV, respectively. Strain is defined as the percentage of systolic shortening of the free wall of the RV from the base to the apex, while strain rate is the velocity with which such shortening takes place.24 Both parameters offer a good correlation with myocardial contractility,34 though strain is less influenced by heart motion and is therefore considered to be more reliable – though it is dependent upon the loading conditions. The strain values should be measured from the apical 4C window without angulation and with high-quality imaging since reverberation and artifacts can affect the placement of the reference points and cause their quantification to be underestimated. The reference points should be limited to the myocardial free wall excluding the pericardium, which may prove difficult since the free wall of the RV is usually thin. A value > –20% is considered normal24 (Fig. 3E).
- o
A pathological value has prognostic implications in patients with normal LV function.35 It is also associated with greater mortality in septic shock (SS),2,36 PHT,37 patients with LV circulatory assist measures38 and COVID-19 cases.39
- -
Speckle-tracking echocardiography. An analysis is made of speckle motion in the two-dimensional image; it is independent of the Doppler angle, with the possibility of quantifying the dynamics of thickening, shortening and rotation of cardiac function.24,40–42 Few studies on critical patients have been published, however.
- -
Three-dimensional echocardiography (ECO-3D). This technique offers a more precise measure of the volumes and RVEF, though the quantified volumes are slightly inferior to those recorded with MRI.43,44 Availability in critical patients is limited.
During their evolutive course, a great variety of cardiological disorders (both primary and secondary) are characterized by diastolic alterations of the RV (myocardiopathies, LV valve diseases, congenital heart disorders, rheumatoid arthritis, vasculitis, ARDS), though there is currently no consensus regarding their evaluation and quantification, and they do not form part of standard clinical echocardiographic assessment.
Recently, guidelines have been published13 for their evaluation based on four parameters (Table 3):
- •
Two-dimensional (2D) imaging. Increase in volume of the RA, dilatation of the IVC with a decrease in inspiratory collapsibility index (CI) of the IVC and myocardial thickening of the RV.
- •
Quantification via PW of the tricuspid inlet flow in the zone of maximum tricuspid leaflet opening. Determination is made of the E wave, A wave, E/A ratio and E wave deceleration time (EDT). This flow is strongly dependent upon preload, postload and the respiratory phase, and determination of the mean of 5 cycles is advised. On the other hand, the coexistence of moderate or severe TI, or atrial fibrillation, underestimates the measurements obtained.
- •
DTI at the lateral tricuspid annulus. This is less dependent upon preload and postload than PW. We obtain the isovolumetric relaxation time (IVRT), eʹ wave, aʹ wave and the eʹ/ aʹ ratio (Fig. 3C and D).
- •
PW in the suprahepatic vein. This is strongly dependent upon the respiratory cycle, MV and positive end-expiratory pressure (PEEP), and determination of the mean of 5 cycles is advised. Three waves are obtained: an anterograde systolic wave (S), caused by relaxation of the RA; an anterograde diastolic wave (D), during rapid ventricular filling; and a reverse flow wave (AR) during the atrial systole.
Right ventricle diastolic function grades.
| Parameters | Normal | Abnormal | Restrictive |
|---|---|---|---|
| ≤ 5 mmDmax IVC ≤ 21 mm andICIVC > 50% | > 5 mmDmax IVC > 21 mm and ICIVC > 50% | |
| 0.8–2.1120–229 | < 0.8> 229 | > 2.1< 120 |
| ≥ 1≤ 6≤ 73 | < 1> 6> 73 | |
| ≥ 1 | < 1 | < 1 and reverse wave |
ICIVC: Index of respiratory collapse of the IVC; Dmax: maximum diameter; IVC: inferior vena cava; E/A: tricuspid filling waves; EDT: E wave deceleration time; ms: millisecond; E/eʹ: ratio between tricuspid flow E wave and lateral eʹ wave of TDI in the lateral tricuspid annulus; IVRT: isovolumetric relaxation time; RV: right ventricle; S wave: systolic wave of hepatic venous flow; D wave: diastolic wave of hepatic venous flow.
As in the LV, there is no echocardiographic parameter capable of isolatedly indicating the existence or grade of diastolic dysfunction of the RV. Instead, we must integrate all the determinations, and the guides recommend classifying the situation as normal or abnormal, with an evolution towards restriction in some disease conditions.
Hemodynamic monitoringIt should be mentioned that for the quantification of cardiac output (CO) and pulmonary pressures or central venous pressure (CVP), we need to place a pulmonary artery catheter or central venous catheter, respectively. However, transthoracic echocardiography (TTE) allows us to estimate these parameters on a noninvasive basis, since due to the anatomy and retrosternal position involved, a poorer analysis is afforded by transesophageal echocardiography (TEE).25 The echocardiographic parameters are shown in Fig. 4 and Table 4.
A. Variation of the inferior vena cava. The 2D inspiration and expiration image is shown in M mode. B. Continuous Doppler recording of tricuspid insufficiency. The red arrow indicates tricuspid insufficiency maximum velocity. C. Isovolumetric relaxation time (IRT). D. Pulmonary artery acceleration time (Tac). E. Pulsed Doppler recording of pulmonary insufficiency. The arrows indicate the protodiastolic maximum velocity of the pulmonary insufficiency flow (VPIed) and the end-diastolic maximum velocity of the pulmonary insufficiency flow (VPIed). F. Normal type I pulmonary artery flow with symmetrical ascent and descent. G. Type I pulmonary artery flow with symmetrical triangular follow, normal flow. H. Type II pulmonary artery flow with asymmetrical triangular follow, suggestive of increased pulmonary pressure. I. Type III pulmonary artery flow with an arrow indicating the mesosystolic notch due to early closure of the pulmonary valve.
Echocardiographic parameters for hemodynamic quantification of the right-side cavities.
| Estimated parameter and echocardiographic parameter | Quantification | Characteristics |
|---|---|---|
Right atrial pressure (RAP)
| ICIVC = (DmaxIVC – DminIVC/DmaxIVC) x 100RAP = 1.62 E/eʹ + 2.13D wave > S waveAbsence of systolic flow wave |
|
Systolic pulmonary artery pressure (PAPs)
| PAPs = 4x (Vmax TI)2 + RAPIVRT | IVRT > 59 ms predicts PAPs > 40 mmHg IVRT < 40 ms excludes PAPs < 40 mmHg (PPV 100%) Tac > 120 ms is normal Tac < 100 ms suggests PHT 60/60 sign is Tac < 60 ms with Vmax TI ≤ 60 mmHg associated with PAPs > 60 mmHg |
| Diastolic pulmonary artery pressure (PAPd) | PAPd = 4x (VPIed)2 + RAP | |
Mean pulmonary artery pressure (PAPm)
| PAPm = 4x(VPIpd)2 + RAPPAPm = 0.61x (VmaxTI)2 + 2 mmHg If Tac < 120 ms, PAPm = 90 – (0.62 x Tac) | Tac < 100 ms predicts PAPm > 25 mmHg |
| Pulmonary vascular resistance (PVR) | PVR = 10 x (VmaxTI/VTIRVOT) + 0.16. |
RAP: right atrial pressure; IVC: inferior vena cava; ICIVC: Index of collapse of the IVC; Dmax: maximum diameter; Dmin: minimum diameter; D wave: diastolic wave; S wave: systolic wave; PAPs: systolic pulmonary artery pressure; PAPd: diastolic pulmonary artery pressure; PAPm: mean pulmonary artery pressure; TI: tricuspid insufficiency; PI: pulmonary insufficiency; Tac: pulmonary artery acceleration time; VmaxTI: Maximum velocity of tricuspid insufficiency; DTI: tissue Doppler imaging; IVRT: isovolumetric relaxation time; VPIpd protodiastolic velocity of the pulmonary artery; VPIed: end-diastolic velocity of the pulmonary artery; VTIRVOT: velocity-time integral of the right ventricle outflow tract.
Right atrial pressure is determined from CVP, and it can be estimated using the following parameters:
- •
Collapsibility index (CI) of the inferior vena cava (IVC). The IVC is measured in the subcostal plane at 1–2 cm from its access to the RA, behind the hepatic vein, with the patient in the supine position and at the end of expiration. Formula: CI = [Dmax IVC - Dmin IVC]/Dmax IVC). In critically ill patients there are situations where dilatation of the IVC without respiratory collapse does not predict the response to crystalloid administration45 (Fig. 4A).
- •
Tricuspid E/eʹ ratio. Registry via PW is made of the protodiastolic filling velocity at tricuspid valve level (wave E), and DTI is used to record the relaxation rate of the lateral wall of the tricuspid annulus in protodiastole (eʹ wave). An E/eʹ ratio > 6 has been shown to be associated to high RAP: > 10 mmHg.25 It has been validated in ventilated patients.13
- •
Hepatic vein flow pattern: An S < D wave ratio is associated with high RAP.13,25
- •
Interatrial septum. Displacement towards the left atrium (LA) is associated with high RAP values.
Systolic, diastolic and mean PAP can be estimated from TI or pulmonary insufficiency (PI) flow using the simplified Bernoulli equation in the absence of pulmonary stenosis, and RAP is added to the calculated gradient.
Systolic pulmonary artery pressure (PAPs)Color Doppler is used to record TI, placing the continuous wave (CW) Doppler cursor with good alignment to calculate the maximum velocity of TI (VmaxTI), which is equivalent to the pressure gradient between the RA and RV. In situations of massive TI, the formula is not applicable, since the inertial component is not negligible. PAPs = 4 x VmaxTI2 + RAP (Fig. 4B).
In patients without detectable TI, estimation can be made via:
- •
IVRT. We position DTI at the level of the free wall of the RV in the tricuspid valve (Fig. 4C).
- •
Pulmonary artery acceleration time (Tac), which is the interval from the start of ejection of the RV to the peak flow velocity through the pulmonary valve (Fig. 4D–G). The 60/60 sign is associated to Tac < 60 with a tricuspid systolic gradient > 30, but < 60 mmHg.
Calculation is made of the peak velocity of PI in end-diastole (VPIed) in the short parasternal axis plane at the large vessel level (Fig. 4E). This method is imprecise in the presence of massive TI.
Mean pulmonary artery pressure (PAPm)Several methods have been described for estimating PAPm:
Pulmonary vascular resistance (PVR)Quantitative measurement of pulmonary vascular resistance (PVR) requires us to relate two measurements: VmaxTI via CW Doppler and the velocity-time integral of the right ventricle outflow tract (VTIRVOT) via PW (image). Quantification is made in Woods units. Formula: PVR = VmaxTI / VTIRVOT x 10 + 0.16.
We can also evaluate PVR in a semi-quantitative manner observing the presence of “notches” in the PW spectrum of the flow velocity in the right ventricle outflow tract (Fig. 4F–H). A mesosystolic notch is indicative of severe PHT (Fig. 4I).
Clinical applications in intensive careAcute dysfunction of the RV is a heterogeneous syndrome resulting from RV-PA decoupling secondary to disorders that have a high incidence in critically ill patients.4 Such decoupling is generally seen in cases characterized by a rapid increase in PAP, in situations of end-stage PHT and in patients with mild PAP presenting inflammatory conditions such as ARDS, sepsis and left ventricular failure – all these disorders also being associated with negative inotropic effects. Furthermore, in many of these scenarios, the patients are subjected to MV, which in itself may intensify or even cause RV failure by inducing an increase in PVR. When MV is applied in patients without cardiorespiratory disease, the tidal volume has no deleterious hemodynamic consequences. However, in the presence of lung injury, the rise in transpulmonary pressure increases RV afterload and reduces Tac (Fig. 4G).
Precapillary vs postcapillary pulmonary hypertensionThe most common cause of RV failure is PHT, defined as PAPm ≥ 25 mmHg.29 The analysis of TI allows us to estimate PHT, with VmaxTI ≤ 2.8 m/s being associated with a low probability of PHT, while VmaxTI > 3.4 m/s is associated with a high probability of PHT (Fig. 5).
Algorithm to determine the probability of pulmonary hypertension based on echocardiography. A the presence of signs of at least 2 categories must be present. b Collapse < 50% with forced inspiration, < 20% with normal inspiration. **RV: Right ventricle; LV: Left ventricle; TAPSE: tricuspid annular plane systolic excursion; PAPs: pulmonary artery systolic pressure; RVOT: right ventricle outflow tract; PA: pulmonary artery; RA: right atrium; IVC: inferior vena cava; PHT: pulmonary hypertension.
Diagnosing the cause of PHT is crucial to define the appropriate treatment. In this regard, a first step is to determine whether the underlying cause is precapillary, with pulmonary capillary pressure (PCP) (wedge pressure) ≤ 15 mmHg, or postcapillary due to pathology of the LV, with PCP ≥ 15 mmHg. The echocardiographic findings suggestive of a pre- or postcapillary etiology are described in Fig. 6.29 The calculation of PVR contributes to establishing the differentiation.30
Echocardiographic signs suggestive of pre- and postcapillary pulmonary hypertension. The blue arrow indicates the interatrial septum bulging towards the left atrium. LA: left atrium; ePLAR: echographic pulmonary to left atrial ratio = [VmaxTI/(E/eʹ) mitral]; PHT: pulmonary hypertension; RVOT: right ventricle outflow tract; LV: left ventricle.
An invasive parameter that has been shown to be useful in differentiating between pre- and postcapillary PHT is the transpulmonary gradient, defined as the difference between PAPm and the pressure of the LA, estimated from the PCP.46 A value of > 12 mmHg is indicative of a precapillary origin. Recently, a surrogate indicator of the transpulmonary gradient has been proposed in the form of the echographic pulmonary to left atrial ratio (ePLAR) measured from the relationship between VmaxTI, as an estimate of pulmonary pressure, and the mitral E/eʹ ratio, as an estimate of the pressure of the LA. In this regard, ePLAR > 0.30 m/s would be indicative of precapillary PHT, while < 0.25 m/s would be indicative of postcapillary PHT.46
Acute and chronic cor pulmonalePrecapillary PHT secondary to lung disease, hypoxia or pulmonary vascular occlusion is classically referred to as cor pulmonale. Depending on the evolution of the increase in RV afterload, a distinction is made between acute and chronic cor pulmonale. In this respect, acute cor pulmonale (ACP) can be secondary to ARDS or PTE, and is defined by the presence of dilatation of the RV quantified by an EDA RV/ EDA LV ratio > 0.6 accompanied by paradoxical motion of the IVS.47 The chronic form of cor pulmonale (CCP) in turn is mainly due to chronic obstructive pulmonary disease (COPD), lung fibrosis or chronic PTE. All of these conditions induce chronic hypoxemia and/or remodeling of the pulmonary circulation,48 requiring the RV to adapt in compensation because of the increase in mechanical effort involved.
Distinguishing ACP from CCP is a challenge in critical patients, and is mainly based on the clinical history and the findings of the clinical examination. It is difficult to know whether echocardiography alone can differentiate between the two conditions, and the existing evidence is scarce and sometimes contradictory.
In general, when the RV afterload experiences an acute increase, the consequences are dilatation and impaired function, while if the pressure increase is gradual, the RV has time to adapt, and remodeling and hypertrophy are more likely to occur.49 However, the thickness of the free wall of the RV can double in 48 hours after the increase in afterload, and so the above is not entirely specific.49
Several specific patterns may help in establishing a distinction, as in the case of acute PTE, where a clot in transit may be observed in the RA or RV, and even in the main trunk of the pulmonary artery. In general, the RV is unable to generate high pressure against acute increases in afterload; consequently, PAPs > 60 mmHg are more suggestive of a chronic process. The 60/60 sign and the McConnell sign, described as a relatively hyperkinetic RV vertex versus a hypokinetic or akinetic RV free wall, have a high positive predictive value (PPV) in the diagnosis of acute PTE.50
The global longitudinal strain of the LV can help distinguish between the chronic and the acute form of cor pulmonale.51,52 In the acute context, it is altered mainly due to regional impairment of the septal, apical and lateral segments, while in the chronic presentation, the global longitudinal strain of the LV is preserved, with only minimal septal involvement.
Acute respiratory distress syndrome (ARDS)It is estimated that 21% of all patients with moderate to severe ARDS will present ACP,52 and this in turn is associated with increased mortality.53 Taking into account that pneumonia as a cause of ARDS, a driving pressure ≥ 18 cmH2O, a PaO2/FiO2 < 150 mmHg and a PaCO2 ≥ 48 mmHg have all been identified as risk factors for the development of ACP in patients with non-COVID-19 ARDS (N-ARDS), a score has been developed allowing the early identification of those patients at risk of presenting ACP and who should be subjected to echocardiographic follow-up.8,52
In patients with ARDS secondary to COVID-19 (C-ARDS), the incidence of ACP is estimated to be 18–38%.54,55 However, the published studies indicate that in COVID-19 patients, the ACP risk score lacks validity, since in the context of C-ARDS the main mechanism associated with the development of ACP is the presence of thromboembolic phenomena in the pulmonary blood vessels. Thus, the finding of ACP in C-ARDS patients is an indication for performing computed tomography to discard the presence of PTE.
In ACP, the RV, before undergoing dilatation via homeometric adaptation to the afterload, increases its contractility to preserve ventricular-atrial coupling. Although the measurement gold standard is Ees/Ea, echocardiographic assessment based on the TAPSE/PAPs ratio has been shown to be a clinically relevant and reliable surrogate parameter of invasive Ees/Ea measurements56(Fig. 2). Recent studies have shown that in both C-ARDS and N-ARDS, early RV-PA decoupling takes place and could be related to the posterior development of ACP and patient mortality.57,58
Septic shockRight ventricle dysfunction affects 35% of all patients with septic shock (SS), and its underlying etiology is multifactorial, since it may be caused by an increase in preload due to excessive fluid therapy, direct myocardial involvement, or an increase in afterload secondary to hypoxemia, hypercapnia or the application of MV.
Right ventricle dysfunction is correlated to mortality, with a more than two-fold increase in mortality risk over both the short and long term.59 Such patients have lower CO and SvO2 values than patients without dysfunction, requiring higher doses of vasoactive drugs and fluid therapy.60 It should be noted that the latter may be a cause or consequence of the situation since the presence of RV dysfunction induces false-positive results in the volume response tests such as the variability of systolic (stroke) volume or pulse pressure, leading to the erroneous administration of fluid therapy and hence a positive fluid balance.61
Although there are validated cut-off points for RV dysfunction,25 their applicability is limited in patients with SS, since these parameters must be contextualized under the conditions of pre- and afterload that can be dynamically altered due to the use of vasopressors, MV and eventually ARDS. Table 5 shows the main echocardiographic studies and the parameters used to define RV dysfunction.
Studies on the relationship between right ventricular dysfunction and mortality in sepsis/septic shock.
| Author/year | Inclusion criterion | N | Echography time | Right ventricular dysfunction criterion | Incidence |
|---|---|---|---|---|---|
| Cirulis,20 2018 | Severe sepsis/septic shock | 146 | 48 h | EDD RV/LV > 0.9 | 55.5% |
| Furian,21 2012 | Severe sepsis/septic shock | 45 | 24 h | RV-Sʹ < 12 cm/s | 31.1% |
| Geri,22 2019 | Post hoc analysis of hemosepsis and HemoPred | 360 | 12 h | EDA RV/LV > 0.8 | 22.5% |
| Mokart,23 2007 | Septic shock | 45 | 1 day | EDD RV > 30 mm + septal dyskinesia, visual RV > LV + PAPs > 45 mmHg | 37.8% |
| Ordre,24 2014 | Severe sepsis/septic shock | 60 | 24 h | ASE criteriaa | 71.6% |
| Pulido,25 2012 | Severe sepsis/septic shock with TTE < 24 h | 71 | 24 h | RV-Sʹ< 15 cm/s, RV/LV ratio, regional contractility alterations, expert opinion | 46.5% |
| Vallabhajosyula,26 2017 | Severe sepsis/septic shock with TTE < 72 h | 388 | 72 h | ASE criteriaa | 55.1% |
N: number of patients; EDD: end-diastolic diameter; RV: right ventricle; LV: left ventricle; Sʹ: tissue Doppler systolic wave; EDA: end-diastolic area; PAPs: systolic pulmonary artery pressure; ASE: American Society of Echocardiography; TTE: transthoracic echocardiography.
A recent meta-analysis of RV dysfunction and mortality in patients with SS found only TAPSE < 16 mm to be associated with increased mortality.62
Right ventricle ischemiaThe coronary circulation of the RV presents features that distinguish it from that of the LV, with flow in systole and diastole. The outflow tract and anterior wall are irrigated by branches of the right coronary artery (RC) and anterior descending artery (AD), while the inferior wall is irrigated by the posterior descending artery, and the lateral wall by the RC. Up to one-third of all patients with acute myocardial infarction (AMI) secondary to AD lesions can present associated RV dysfunction due to a decrease in flow from the branches of the AD to the anterior wall of the RV and/or involvement of the interventricular septum.13 Right ventricle AMI is usually caused by proximal occlusion of the RC, generally in association with AMI of the lower LV.42 Isolated necrosis of the RV occurs in < 3% of the cases, due either to left-side dominance or to isolated occlusion of branches of the RC.63
In the absence of hypertrophy and/or PHT, the RV is less vulnerable to ischemia than the LV, conditioned by the factors25 described in Table 6. The severity of dysfunction depends on the location of the occlusion of the RC. Considering the aforementioned characteristics of resistance to ischemia, spontaneous recovery from dysfunction is common. Thus, the term AMI of the RV includes a spectrum of conditions associated with transient ischemia, ischemic damage or myocardial necrosis.
Characteristics of the right ventricle that determine its greater ischemic resistance.
| LESS OXYGEN CONSUMPTION, due to lesser thickness of the myocardial wall and contraction against a system of lesser pressure. |
| INCREASED OXYGEN EXTRACTION UNDER ISCHEMIC CONDITIONS, with 50% extraction under resting conditions. |
| GREATER OXYGEN SUPPLY DURING CARDIAC CYCLE, due to homogeneous blood supply from the right coronary artery during systole and diastole. |
| RAPID DEVELOPMENT OF COLLATERALS after occlusion of the right coronary artery, particularly from the moderator band artery - a branch of the anterior descending artery. |
| PRESERVATION OF THE INFUNDIBULUM FROM ISCHEMIA, with 11–30% of its perfusion being derived from an independent artery with its own ostium. |
The following echocardiographic parameters of AMI of the RV have been described:
- •
TAPSE <10 mm has a positive predictive value (PPV) of 75% in diagnosing AMI of the RV (Fig. 3); in the presence of dilatation of the RV this value is not reliable, however, since there is an increase in the radial component.
- •
Color Doppler identifies mechanical complications such as wall rupture, right-to-left shunting through the foramen ovale, or secondary TI due to annular dilatation and/or papillary muscle ischemia.
- •
Sʹ/RIMP. This is a new index that combines the peak of the Sʹ wave measured by DTI in the tricuspid annulus with the right ventricle index of myocardial performance (RIMP), with good sensitivity and specificity in identifying AMI of the RV in patients presenting inferior AMI.64 Specifically, Sʹ/RIMP < 17 is associated with RV dysfunction due to proximal lesions of the RC in the context of inferior AMI, with a sensitivity of 85% and a specificity of 87%.
- •
Strain/strain rate.65 In patients with inferior AMI, the values in basal and middle segments of the RV are lower when associated with involvement of the RV.66
- •
Three-dimensional echocardiography. A RVEF < 51% using ECO-3D in patients with inferior AMI of the LV is correlated to ischemic involvement of the RV.
Involvement of the RV upon admission is associated with a poorer prognosis in patients with AMI and with increased mortality in patients with cardiogenic shock.67 Its dysfunction in patients with inferior AMI increases the number of hospital readmissions when the parameter used is TAPSE < 15 mm,63 Sʹ wave < 8 cm/s68 or longitudinal strain of the RV > 22%.69
ConclusionsUnderstanding the mechanisms of RV damage, in particular RV-PA decoupling, can inform us about the type and timing of the hemodynamic interventions and complementary therapies applied to protect and “resuscitate” the RV22 (Fig. 2). A noninvasive diagnostic and monitoring approach at the patient bedside can help to determine the RV lesion phenotype and understand the response to treatments, with better personalization of care and an improved prognosis for populations with a view to future studies.
The treatment of RV lesions in ARDS includes the use of drugs such as vasoactive agents that improve RV-PA coupling, pulmonary vasodilators to reduce the afterload, and non-pharmacological strategies such as RV protective ventilation,22 the prone position22 and extracorporeal membrane oxygenation (ECMO).70
Conflict of interestThe authors declare that they have no conflicts of interest in relation to any of the topics addressed in this chapter of the Update on “Ultrasound in the Critical Patient. Clinical applications” of the journal Medicina Intensiva. Likewise, no financial support has been received for conducting the study.
The manuscript has been commissioned by the Editorial Board of the journal Medicina Intensiva, and its authors have been selected by the Board.
Lastly, and no less importantly, the authors thank the Editorial Board members of the journal Medicina Intensiva, and particularly the previous Editor-in-Chief, for endorsing the Update on “Ultrasound in the Critical Patient. Clinical applications” and the invitation to prepare this chapter, entitled: “Right ventricular dysfunction in the critically ill. Echocardiographic evaluation”. This is a current topic of great interest in application to critically ill patients, and the chapter offers an analysis of the most relevant developments in scientific evidence over the last two years, with changes in the monitoring techniques used. The authors appreciate the opportunity to present the most appropriate parameters in the echocardiographic study of the right ventricle in these patients based on the scientific evidence, and the changes in the current mechanical ventilation strategies.

















![Echocardiographic signs suggestive of pre- and postcapillary pulmonary hypertension. The blue arrow indicates the interatrial septum bulging towards the left atrium. LA: left atrium; ePLAR: echographic pulmonary to left atrial ratio = [VmaxTI/(E/eʹ) mitral]; PHT: pulmonary hypertension; RVOT: right ventricle outflow tract; LV: left ventricle. Echocardiographic signs suggestive of pre- and postcapillary pulmonary hypertension. The blue arrow indicates the interatrial septum bulging towards the left atrium. LA: left atrium; ePLAR: echographic pulmonary to left atrial ratio = [VmaxTI/(E/eʹ) mitral]; PHT: pulmonary hypertension; RVOT: right ventricle outflow tract; LV: left ventricle.](https://static.elsevier.es/multimedia/21735727/0000004800000009/v1_202409021221/S2173572724001735/v1_202409021221/en/main.assets/thumbnail/gr6.jpeg?xkr=1dZuESKpnCAWr3yCSGZ24A==)


