The SARS-CoV-2 pandemic has made it really difficult to be able to make decisions at the ICU setting due to the lack of primary studies completed and because most preliminary results available are retrospective. For these reasons we agree with Santillán-García1 in that the Living Systematic Review (LSR) strategy or “live evidence” can be a good strategy to improve the decision-making process at the ICU setting during the current pandemic. Defined by Elliot et al.2 back in 2014, the LSR strategy deals with the constant update of systematic reviews by including new relevant evidence as this becomes available.
We present our series of cases collected retrospectively that shows the results derived from implementing this methodology to the clinical decision-making process during the current pandemic.
Back in February of this year, in our hospital, a non-profit cooperative private center of 250 beds, the Hospital de Barcelona COVID-19 Decision Making Working Group (HB-Covidem) was created. Before the first case of COVID-19 was ever reported and following the Guidance for the production and publication of Cochrane living systematic review strategy methodology,3 this working group created a panel of “open recommendations”. Given the scarcity of finished primary studies, consensus documents,4 clinical practice guidelines, and systematic reviews were based on interferences from other clinical settings rather than relevant evidence. For this reason, the open recommendations from this panel were updated daily with more agile and depurated sources like through online publications of preliminary results or SSRN and medRxiv reprints, and registries still unpublished from the Twitter channel @CovidNma. The objective of these open recommendations was to give an agile and effective support to clinicians without losing focus on the directives that need ongoing reviews and updates based on the current epidemiological situation and possible changes made to the therapeutic options.5,6
The UCI multidisciplinary team implemented the therapeutic options available after the individual analysis of every patient admitted with COVID-19 related pneumonia. Decisions were updated twice a day during the clinical sessions held by intensivists, anesthesiologists, and nurses: at 9:00 AM and 21:00 PM. Decisions included options on drugs from the off-label drug armamentarium available and different possibilities regarding oxygen therapy techniques or mechanical ventilation.
The Intellivue Clinical Information Portfolio (ICIP)7 software was used to register and validate the prospective and consecutive case series with COVID-19 as confirmed by the lab and transferred for ICU admission, by the Sistema d’Emergències Mèdiques coordinating center or by any other hospital services. The patients’ demographic and clinical data were gathered including data on complications and mortality. The study ethical and methodological aspects were approved by the Teaching and Research Committee of the SCIAS Hospital de Barcelona.
Between March 7 and May 14, 593 patients were admitted to the hospital with COVID-19 related pneumonia. A total of 61 of these patients (10.2% [95%CI: 8.0%–13.0%]) required admission in some of the 31 beds available of the extended ICU under the sole responsibility of the intensivists. As Table 1 shows, the patients’ median age was over 65 and three fourths of the patients were males. The most common comorbidities were arterial hypertension, diabetes mellitus, and chronic respiratory disease. Regarding inflammatory markers at admission, the median serum ferritin values were >900 ng/mL and the SOFA score was >6 points in 90% of the patients (Table 1).
Demographic and clinical characteristics of patients admitted to the ICU with COVID-19 related pneumonia (n = 61).
| All | Invasive mechanical ventilation | Non-invasive mechanical ventilation | High-flow nasal cannulas | |
|---|---|---|---|---|
| Number (%[95%CI]) | 61 (100) | 21 (34.3 [22.2−44.7]) | 35 (57.5 [44.1−70.0]) | 5 (8.2 [2.7−18.1]) |
| Age, median, years (IQR) | 66 (60−72) | 65 (59−71) | 68 (61−74) | 67 (60−68) |
| Males, n (%[95%CI]) | 53 (86.8 [75.8−94.2]) | 18 (85.7 [63.7−97.0]) | 31 (88.5 [76.9−98.9]) | 4 (80.0 [28.4−99.5]) |
| Women, n (%[95%CI]) | 8 (13.2 [6.8−39.1] | 3 (14.3 [2.0−25.8] | 4 (11.5 [3.2−26.7]) | 1 (20.0 [5.0−71.6]) |
| Comorbidities, n (%[95%CI]) | ||||
| Hypertension | 24 (39.3 [27.1−52.7] | 12 (57.1 [34.0−78.2]) | 10 (28.5 [14.6−46.3]) | 2 (40.0 [5.3−85.3]) |
| Diabetes | 22 (36.0 [24.2−49.4]) | 10 (47.6 [24.8−67.8]) | 11 (50.0 [28.2−71.8]) | 1 (20.0 [5.0−98.0] |
| COPD | 6 (9.8 [3.7−20.2]) | 3 (14.2 [3.0−36.3]) | 2 (5.7 [7.0−19.2]) | 1 (20 [5.0−98.0]) |
| Other | 38 (62.2 [49.0−74.4]) | 21 (100) | 16 (45.7 [27.9−61.9]) | 2 (40 [5.3−85.3] |
| Serum ferritin, ng/mL, median (IQR) | 912 (412−1.240) | 922 (415−1.310) | 899 (398−1.210) | 887 (393−1.190) |
| SOFA, >6 points, n (%[95%CI]) | 55 (90.1 [77.8−95.3]) | 21 (100) | 31 (88.5 [73.3−96.8]) | 4 (80 [28.4−99.5]) |
| Off-label drugs (1): azithromycin, hydroxychloroquine, lopinavir/ritonavir, heparin, n (%[95%CI]) | 59 (96.7 [88.7−99.6]) | 21 (100) | 35 (100) | 3 (60.0 [14.7−94.7]) |
| Off-label drugs (2): methylprednisolone, n (%[95%CI]) | 55 (90.1 [77.8−96.3]) | 21 (100) | 30 (85.7 [69.7−95.2]) | 4 (80 [28.4−99.5]) |
| Off-label drugs (3): tocilizumab, n (%[95%CI]) | 16 (26.2 [18.5−28.0]) | 10 (47.6 [25.7−70.2]) | 6 (17.1 [6.6−36.6]) | |
| Off-label drugs (4): interferon, n (%[95%CI]) | 10 (16.3 [8.2−28.0]) | 6 (28.5 [11.3−52.2]) | 4 (11.4 [3.2−26.7]) | |
| FiO2 > 50%, n (%[95%CI]) | 54 (88.5 [77.8−95.3]) | 21 (100) | 32 (91.4 [76.8−98.2]) | 1 (20 [5.0−71.6]) |
| PaO2/FiO2 (median [IQR]) | 170 (96−218) | 167 (90−210) | 176 (143−228) | 180 (146−230) |
| PEEP (median [IQR]) | 11 (9−15) | 12 (10−16) | 10 (8−14) | ― |
Regarding pharmacological treatment, all patients except for 2 had contraindications and were treated with drugs from the off-label drug armamentarium (azithromycin, hydroxychloroquine, lopinavir/ritonavir, and fragmented heparin) at thromboprophylactic doses without relevant adverse events reported in any of the cases (Table 1). Also, 90% of the cases received methylprednisolone at a dose of 1 mg/kg/day depending on the progression of the ARDS. Also, 16 patients received tocilizumab depending on the results of the inflammatory markers and 10 patients received interferon for the same reason before tocilizumab was included in the protocol (Table 1).
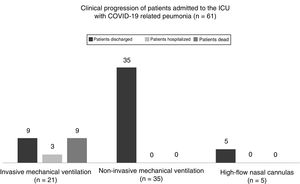
As Table 1 shows regarding ventilatory support, intubation was avoided in 40 patients (65.5% [95%CI: 60.9%–84.2%]). A total of 35 of these patients (57.3% [95%CI: 44.1%–70.0%]) received non-invasive mechanical ventilation for an average 5 days ± 3.0 days (95%CI: 4.0–5.9 days) and 5 patients (8.2% [95%CI: 2.7%–18.1%]) were treated with high-flow nasal cannulas (HFNC) for an average 2.4 days ± 1.9 days (95%CI: 1.1 days–2.8 days). The remaining 21 patients required orotracheal intubation and lung protective mechanical ventilation for an average 16 days ± 15 days (95%CI: 9.5 days–22.4 days) and had to be placed in the decubitus position for an average 5.6 days ± 5.1 days (95%CI: 2.8 days–7.1 days). The median PaO2/FiO2 ratio at admission and the median PEEP and FiO2 levels are shown on Table 1. A total of 3 patients (4.9% [95%CI: 1.0%–13.7%]) were tracheostomized after a median non-invasive mechanical ventilation time of 21 days (IQR = 15 days–26 days).
As Fig. 1 shows, back in March 14, the mortality rate of those admitted to the ICU did not change significantly compared to the overall in-hospital mortality rate since 71 patients died while at the hospital (11.9% [95%CI, 9.5%–14.8%] compared to 9 deaths while at the ICU setting (14.7% [95%CI, 7.0%–26.2%]; P = .527). All of the patients who died would have required orotracheal intubation (the mortality rate of intubated patients is 42.8% [95%CI, 21.8–66.0 %]). However, no deaths were reported among those patients treated with a facial mask or a HFNC.
In our case series of critically ill patients with COVID-19 related pneumonia confirmed by the lab and hospitalized at the extended ICU of the SCIAS Hospital de Barcelona, most patients were males over 60 with critical hypoxemia (PaO2/FiO2 < 200) and high blood pressure who received multiple off-label drugs and in whom, in almost two thirds of them, we managed to avoid intubation using non-invasive mechanical ventilation masks or HFNCs. The overall mortality rate at the ICU setting was <15%, only slightly higher compared to the overall in-hospital mortality rate.
The greatest limitation of our prospective case series is that the sample is small. However, we believe that the results are promising and may open up the possibility of a future multicenter study that should analyze whether an organizational change in the decision-making process at the ICU setting, in the LSR sense, may create a more agile, flexible, effective, and efficient system in emergent health situations8 where decisions have a huge impact on the patients’ survival.9
FundingNone whatsoever.
Conflicts of interestNone reported.
Karla Núñez, Paola Jubert, Elisabet Periche, Eugenia Portillo, Ana Ayestarán, Jaume Llevaría, Alejandra Duarte, Montserrat Vaqué, Mercedes Clemente, Xavier Sanz, Roser Cid, Anna Vilà, Mónica León, Jordi Fabregat, Lucía Ortega, Júlia Pareja, Alejandra Méndez, and Marta Muntané.
Please cite this article as: Carrasco G, Morillas J, Calizaya M, Baeza I, Molina R, Meije Y. Decisiones en UCI basadas en la estrategia Living Systematic Review durante la pandemia de SARS-CoV-2. Resultados de una serie prospectiva de casos. Med Intensiva. 2020;44:517–519.